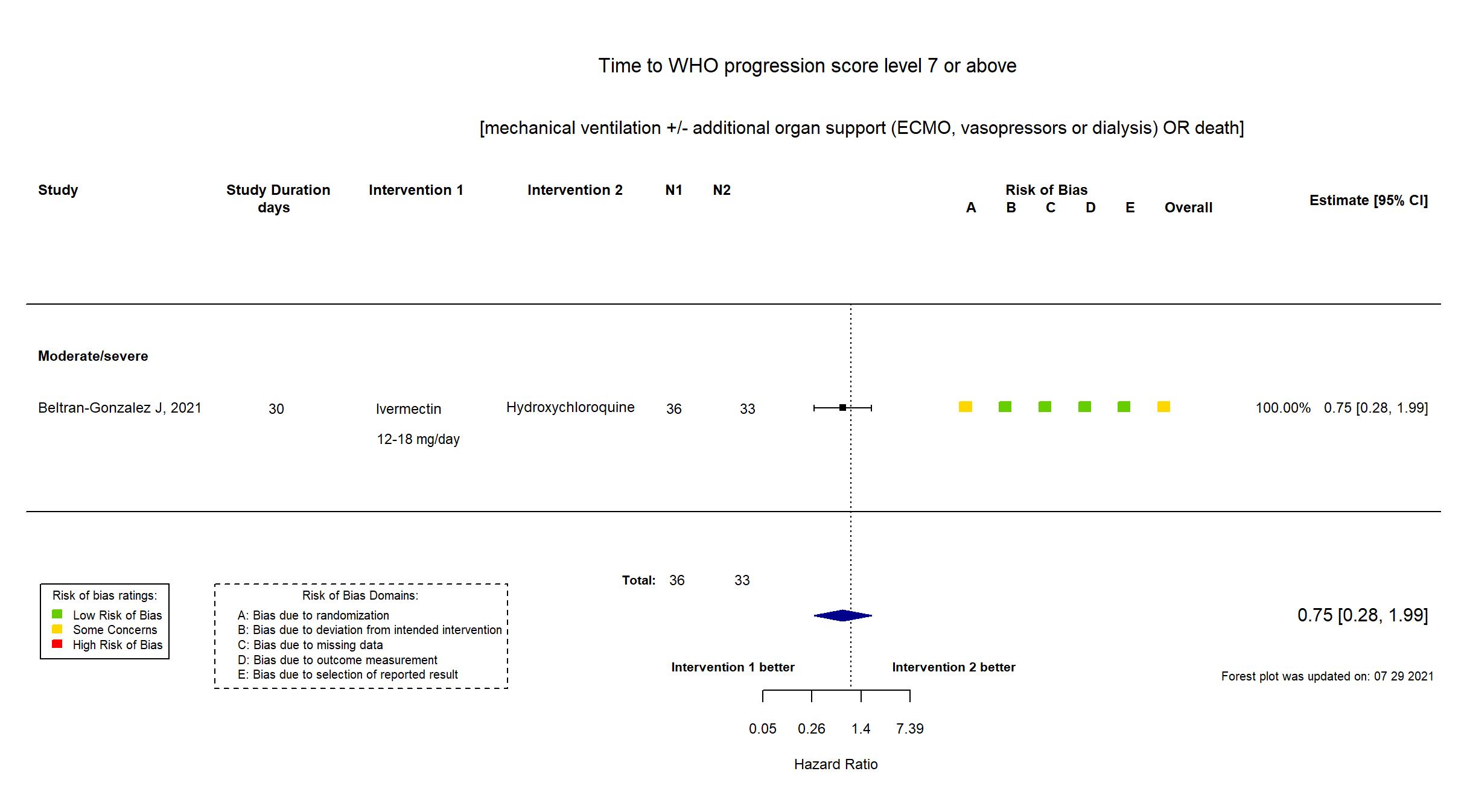
Ivermectin vs Hydroxychloroquine (RCT)
Hospitalized patients
FOREST PLOTS -2021-07-29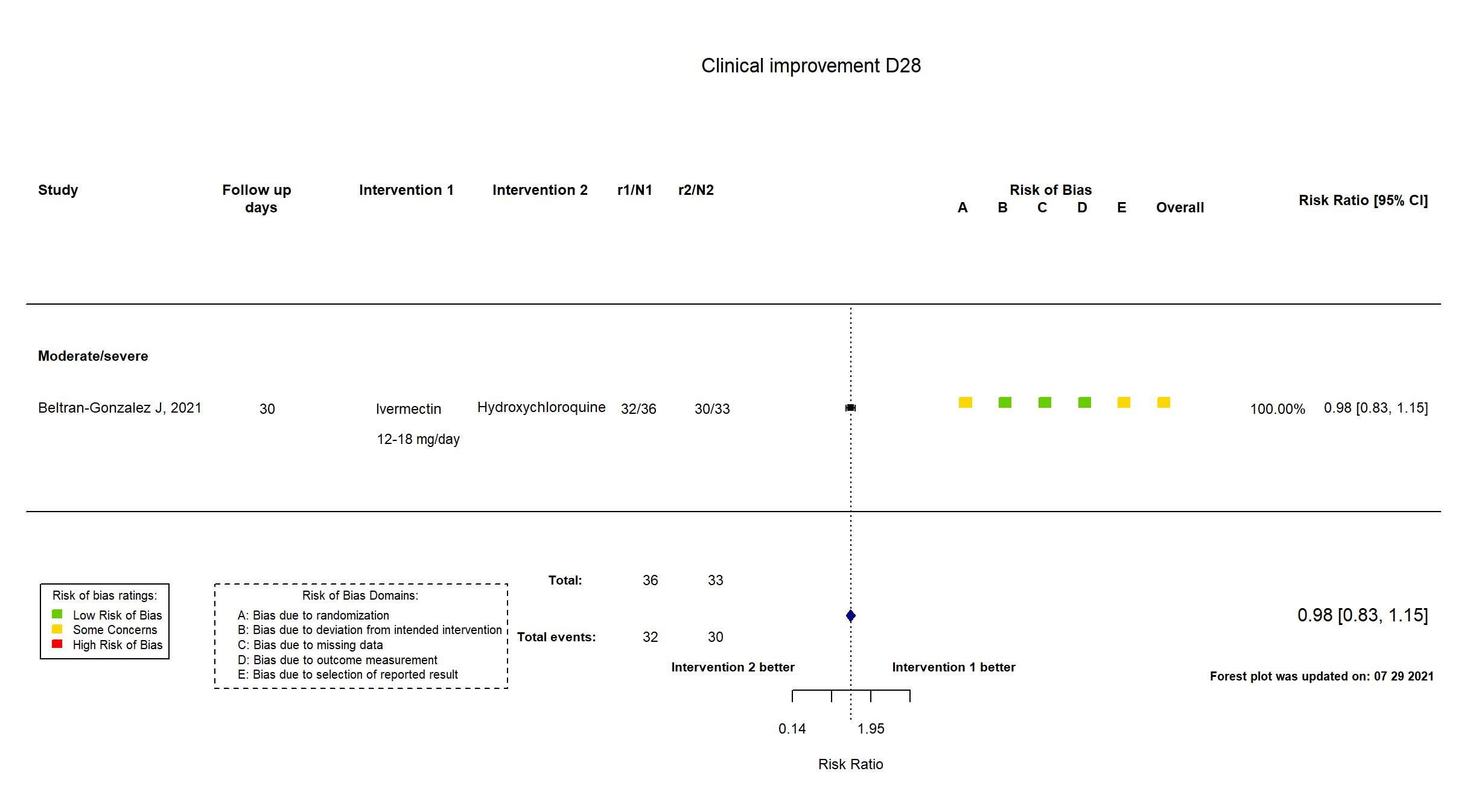
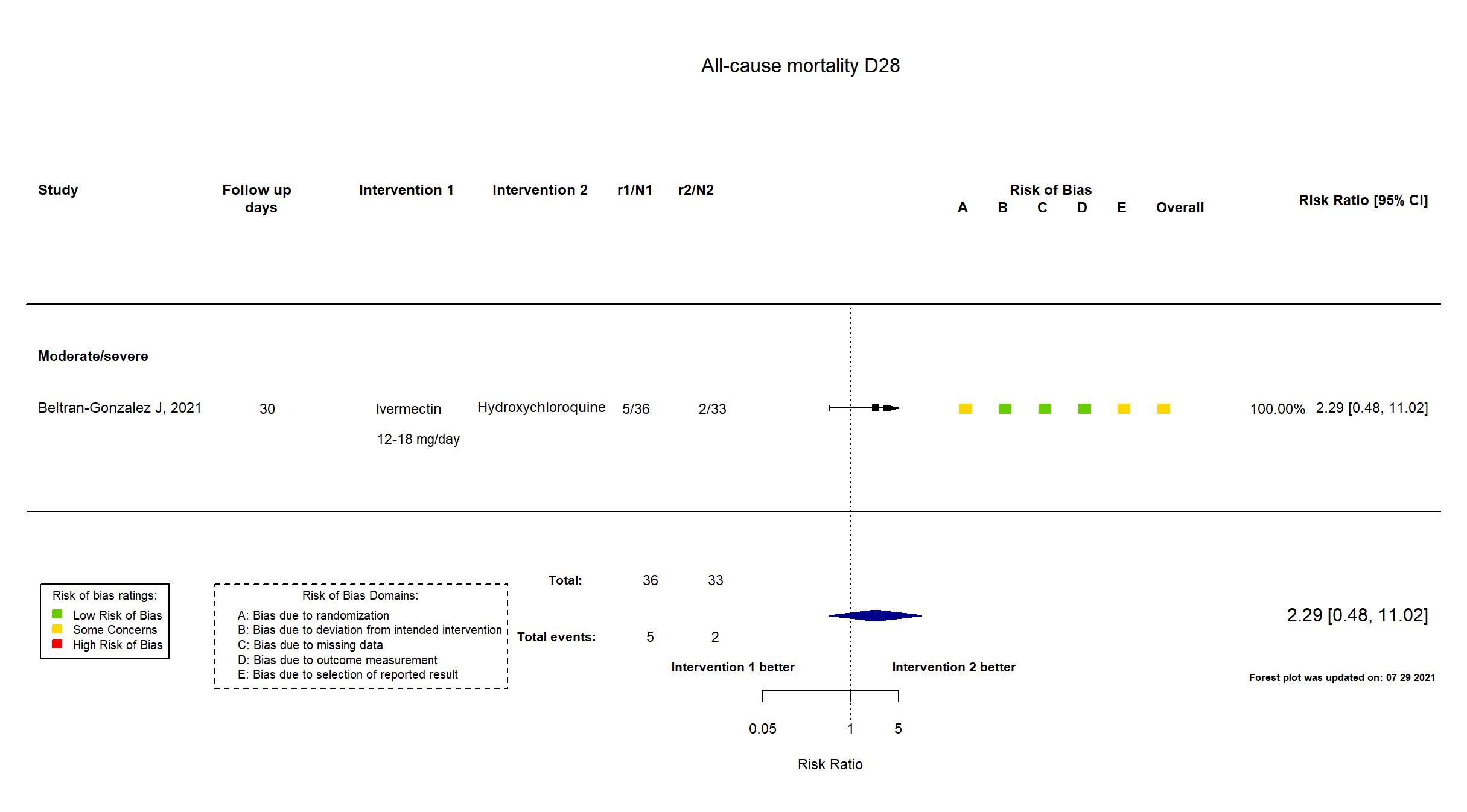
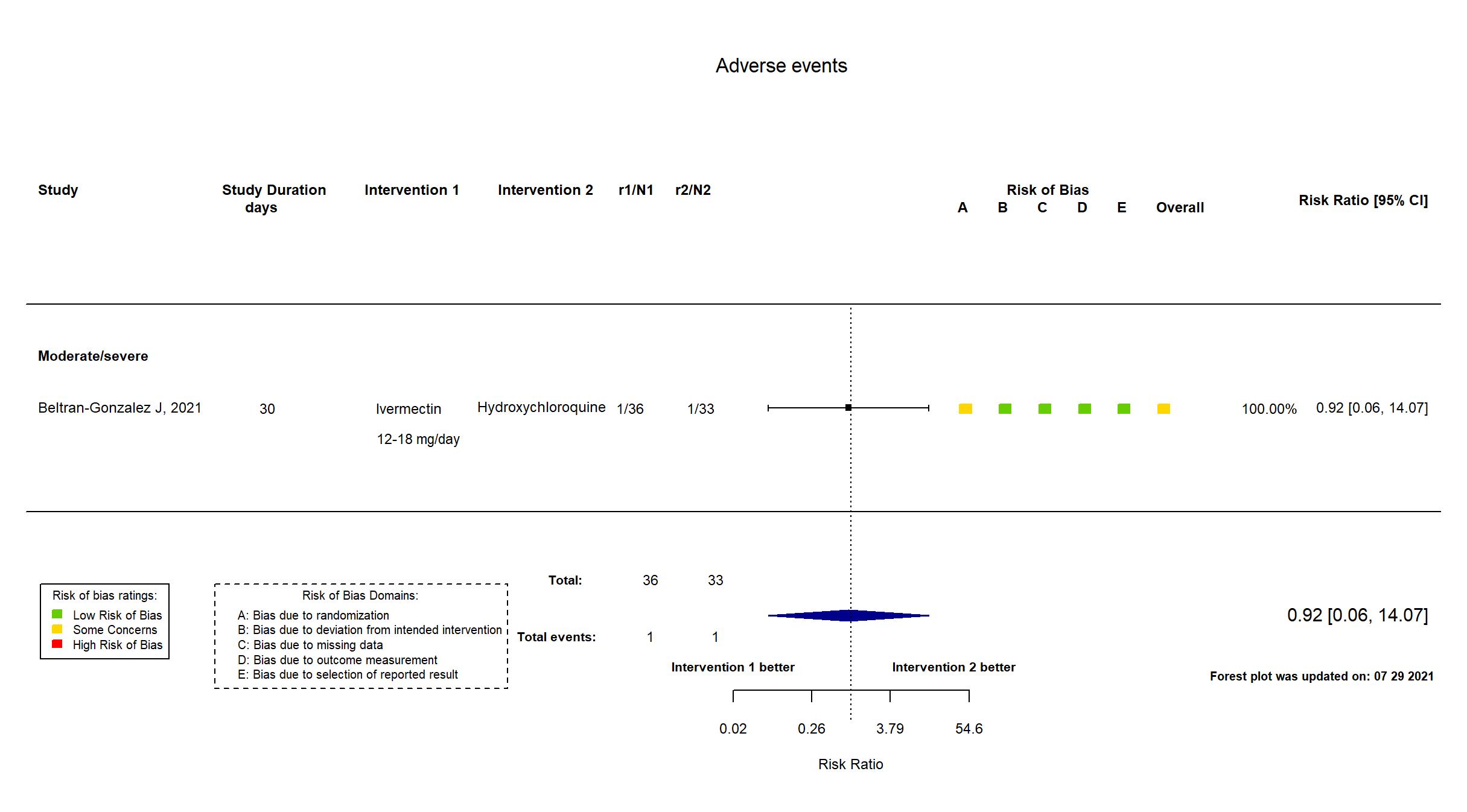
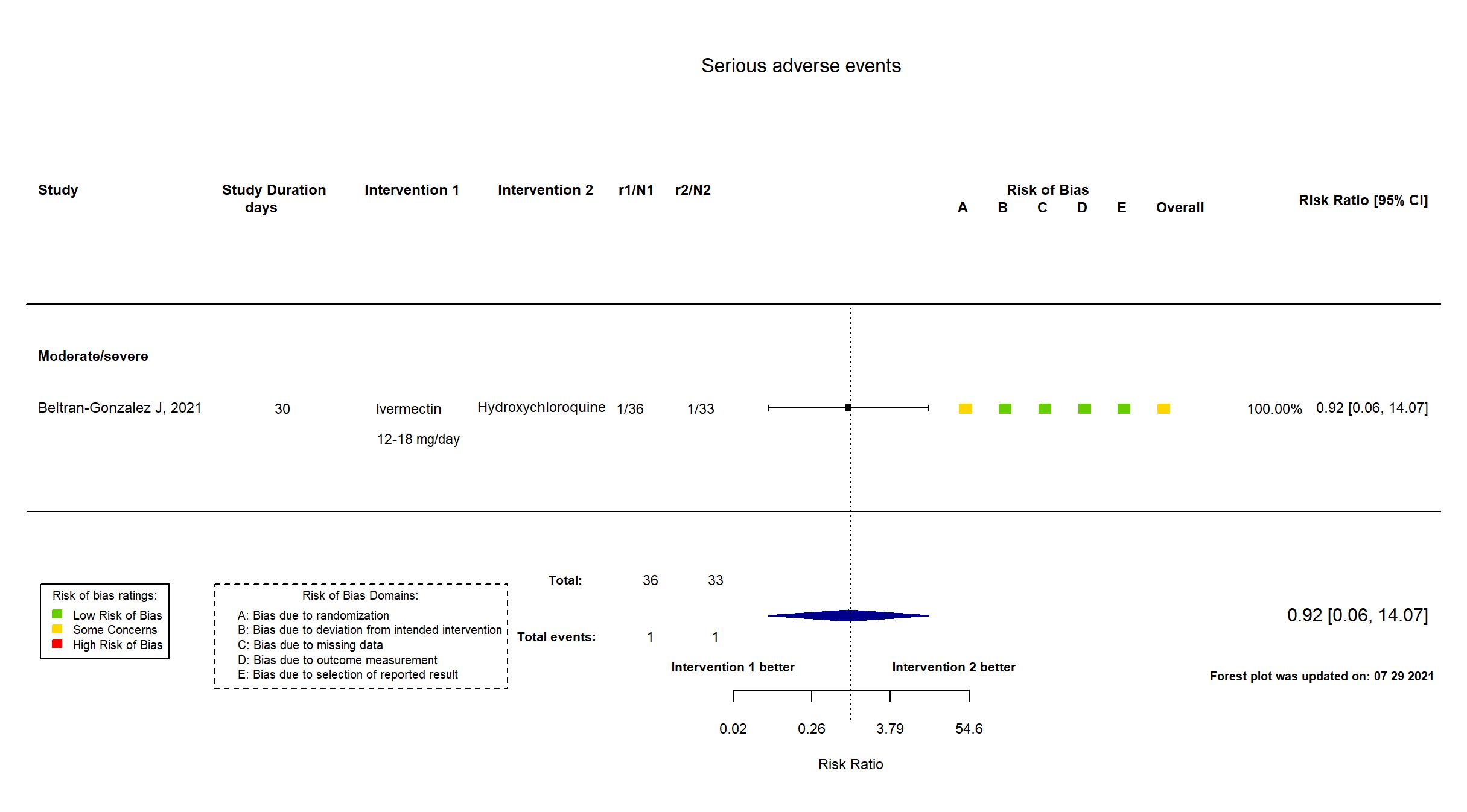





Trial NCT04391127
Publication Beltran-Gonzalez J, medRxiv (2021) (preprint)
Dates: 2020-05-04 to 2020-08-15
Funding: Public/non profit (Aguascalienes State Health Institute)
Conflict of interest: No
| Methods | |
| RCT Blinding: Double blinded, no restrictions | |
| Location :
Single center / Mexico Follow-up duration (days): 30 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Hydroxychloroquine Initial dose: 400 mg orally twice a day for the first day. Maintenance dose: 200 mg orally twice a day for another 4 days. Ivermectin 12 mg for one day in patients weighing < 80 kg and 18 mg for one day in those >80 kg. |
|
| Control
Placebo Two calcium citrate tablets orally twice a day for one day. Subsequently one tablet twice a day for 4 more days. | |
| Participants | |
| Randomized participants : Hydroxychloroquine=33 Ivermectin=36 Placebo=37 | |
| Characteristics of participants N= 106 Mean age : NR 66 males Severity : Mild: n=0 / Moderate: n=16 / Severe: n=90 Critical: n=0 | |
| Primary outcome | |
| In the register Mean days of hospital stay [ Time Frame: Three months ]; Rate of Respiratory deterioration, requirement of invasive mechanical ventilation or dead [ Time Frame: Three months ]; Mean of oxygenation index delta [ Time Frame: Three months ] | |
| In the report Hospitalization duration until discharge due to clinical improvement, the total duration of hospitalization, and the safety outcomes were duration of hospitalization until respiratory deterioration (previously defined) or death | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the pre-print article, the trial registry was used in data extraction and assessment of risk of bias. Neither study protocol nor statistical analysis plan was available. Inclusion criteria in registry and the pre-print article differ slightly in that the pre-print article also included hypoxemic respiratory failure or acute clinical deterioration of pre-existing lung or heart disease. Some pre-stated primary (i.e., mean of oxygenation index delta) and secondary (i.e., mean time to negative PCR) outcomes were not reported. There were no substantive differences between the pre-print article and the trial registry in interventions. Patients considered at high risk of development of QT interval prolongation due to hydroxychloroquine were only randomized to the ivermectin or placebo arms. The trial was terminated due to a reduction in eligible participants. As a result, the target sample size was not achieved.
This trial was updated on July 28th, 2021 with data gained from contact with authors. |
Trial RBR-8h7q82
Publication Galan L , Pathog Glob Health (2021) (published paper)
Funding: Public/non profit (Universidade Federal de Roraima)
Conflict of interest: No
| Methods | |
| RCT Blinding: Participants, outcome assessor and health care pro | |
| Location :
Single center / Brazil Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Hydroxychloroquine Initial dose: 400 mg orally twice on day 0 - Maintenance dose: 400 mg orally once daily twice on day 1, and once daily from day 2 to day 5 Chloroquine Initial dose: 450 mg orally twice on day 0 -Maintenance dose: 450 mg orally twice on day 1, and once daily from day 2 to day 5 |
|
| Control
Ivermectin 14 mg orally once from day 1 to day 3 (+ placebo tablets on days 1, 4 & 5 to maintain blinding) | |
| Participants | |
| Randomized participants : Hydroxychloroquine=54 Chloroquine=61 Ivermectin=53 | |
| Characteristics of participants N= 168 Mean age : NR 95 males Severity : Mild: n=0 / Moderate: n=* / Severe: n=* Critical: n=* | |
| Primary outcome | |
| In the register Need for supplemental oxygen, need for invasive ventilation, need for admission to the intensive care unit (ICU) | |
| In the report Overall survival | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
* |
| General comment | The prospective trial registry was available. There were no differences between the published article and the registry in population or interventions. The study achieved its target sample size. No study protocol or statistical analysis plan was available. Outcomes reported were no relevant for the COVID-19 NMA. Consequently, no outcome data have been extracted for this study. |