Convalescent plasma vs Standard care/Placebo (RCT)
Mild outpatients
Studies included but not extracted/included in the analysis: Jalili E,Tanaffos, 2022; Denkinger C M, medrxiv, 2022 ; Thorlacius-Ussing L, Sci Rep, 2022;
Villanueva C, medrxiv, 2022
;
Muller-Tidow C, Hemasphere, 2022
FOREST PLOTS -2022-11-17
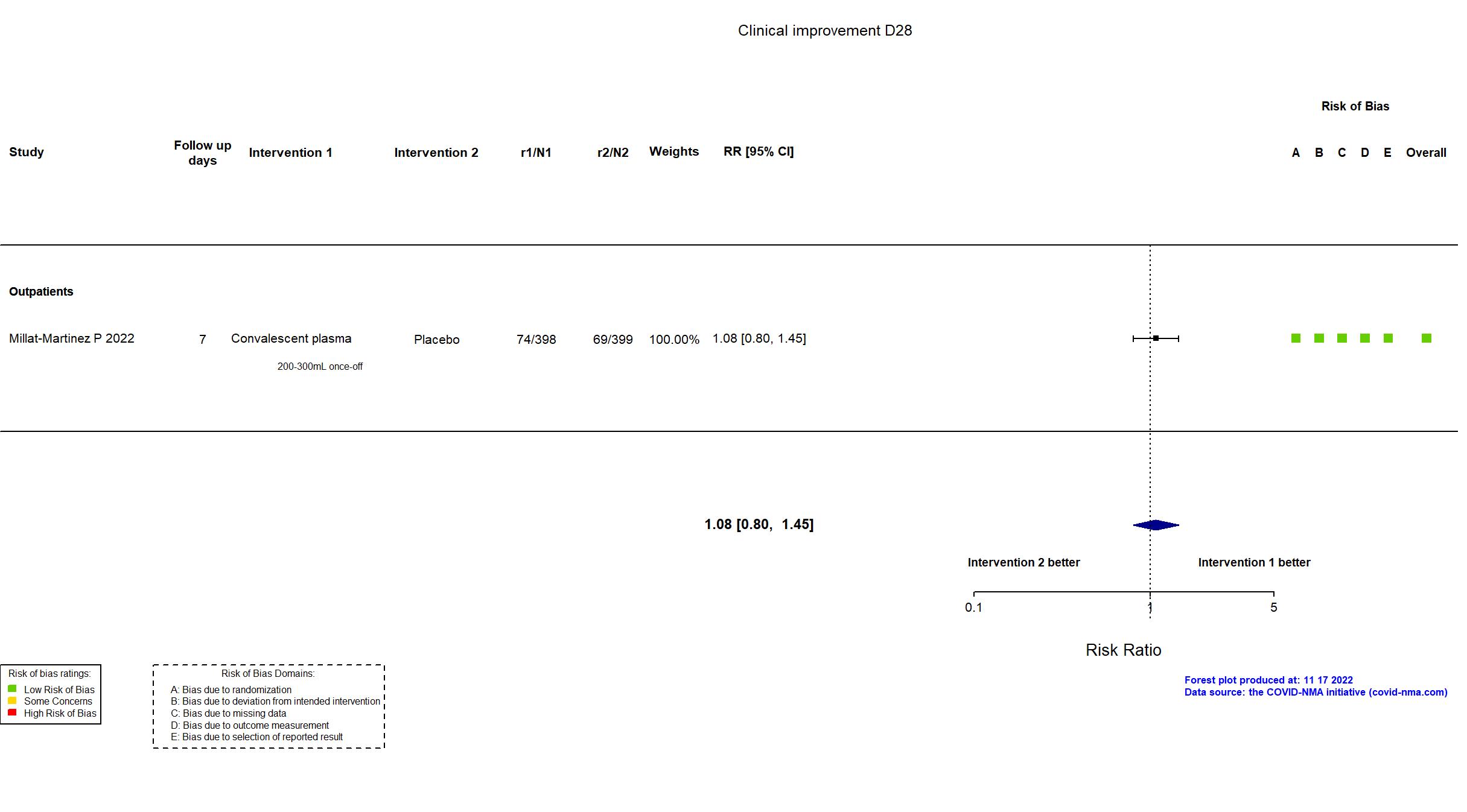
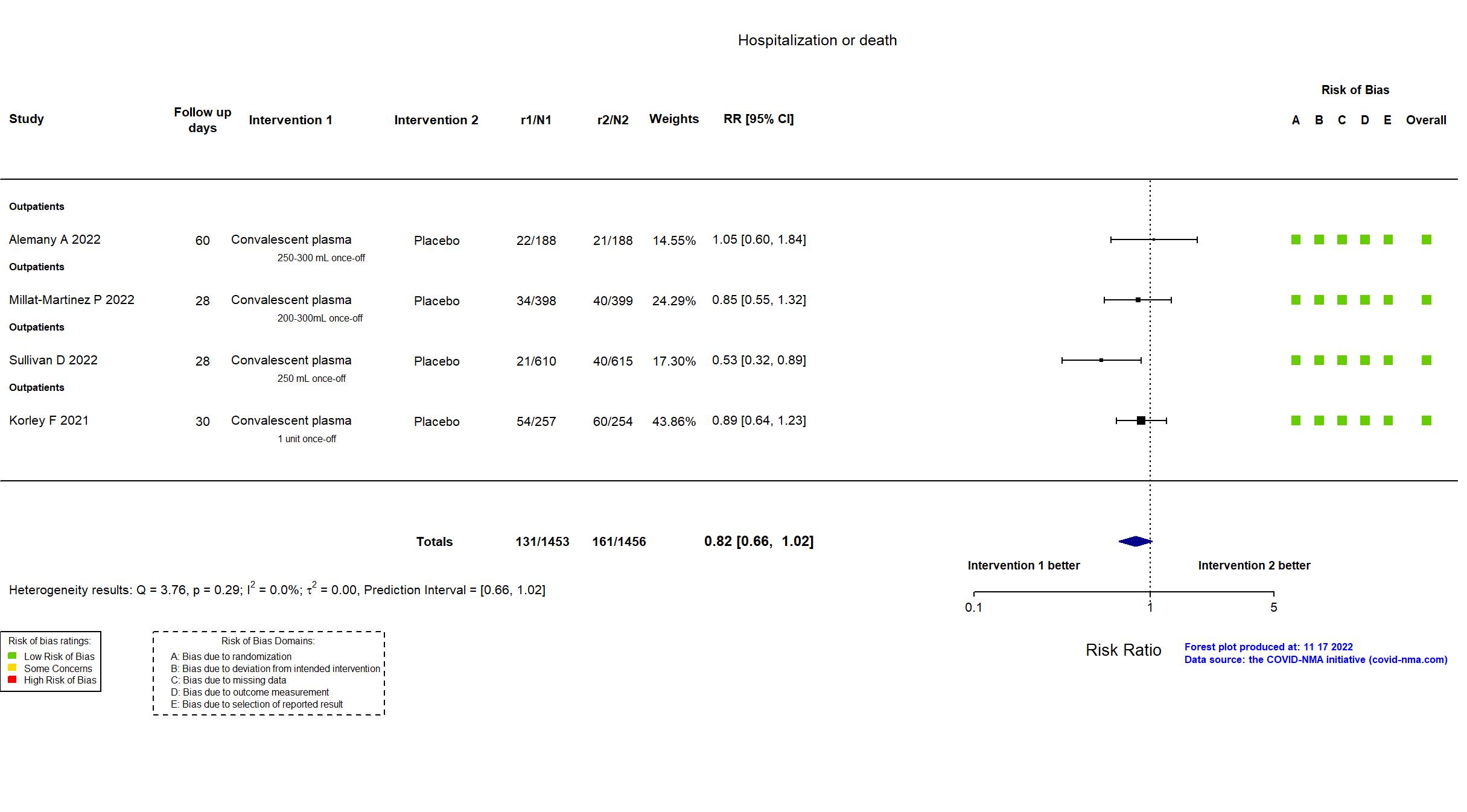
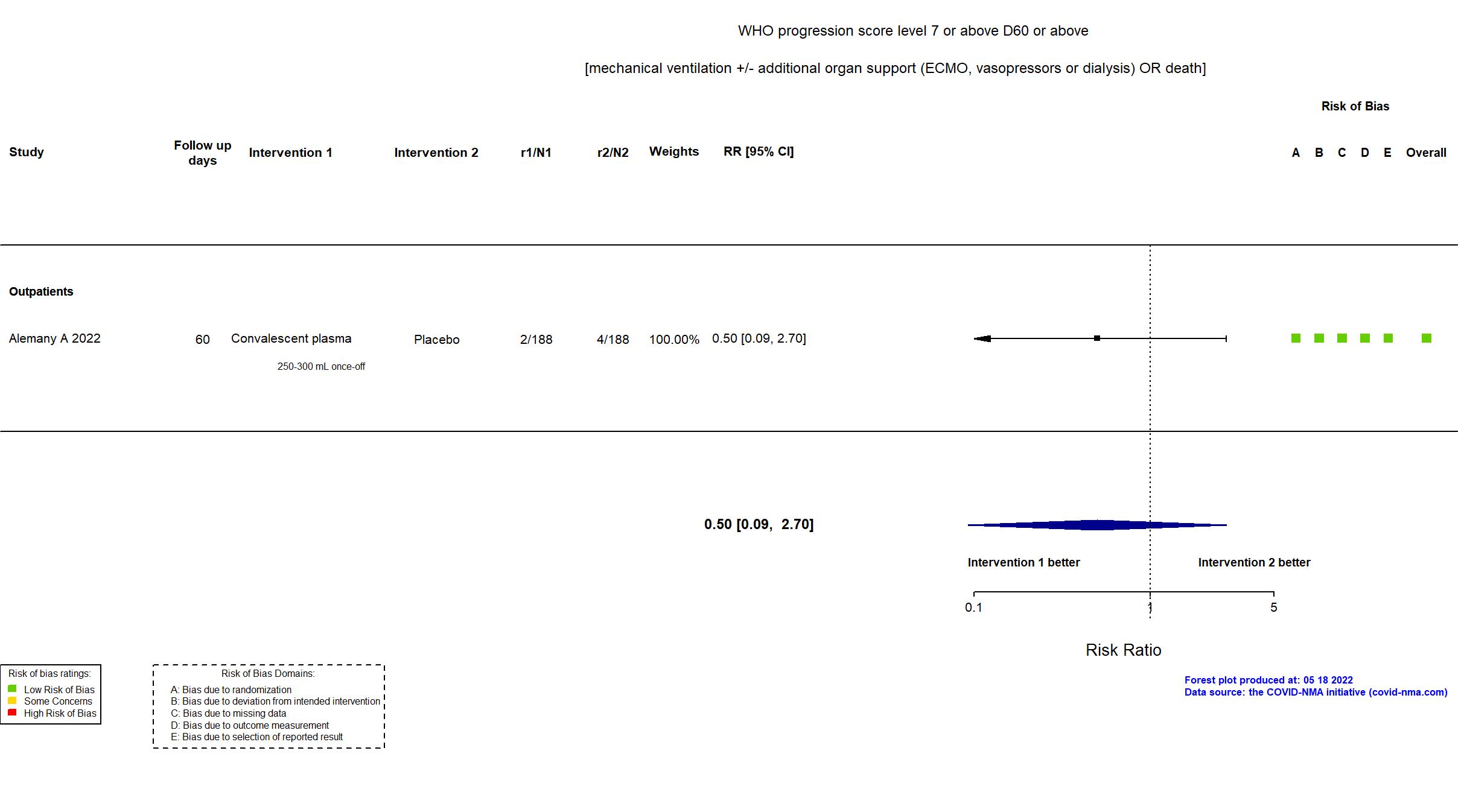
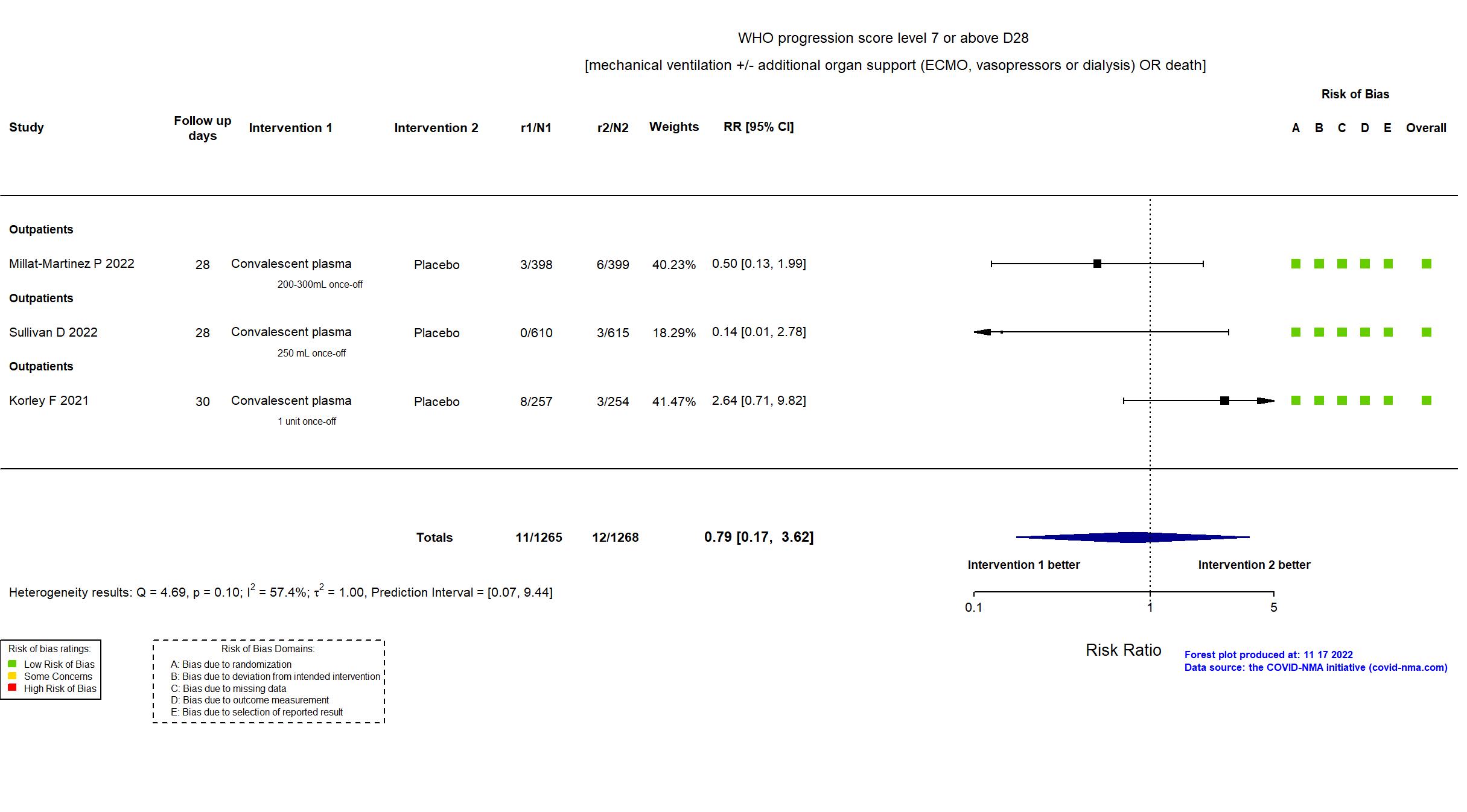
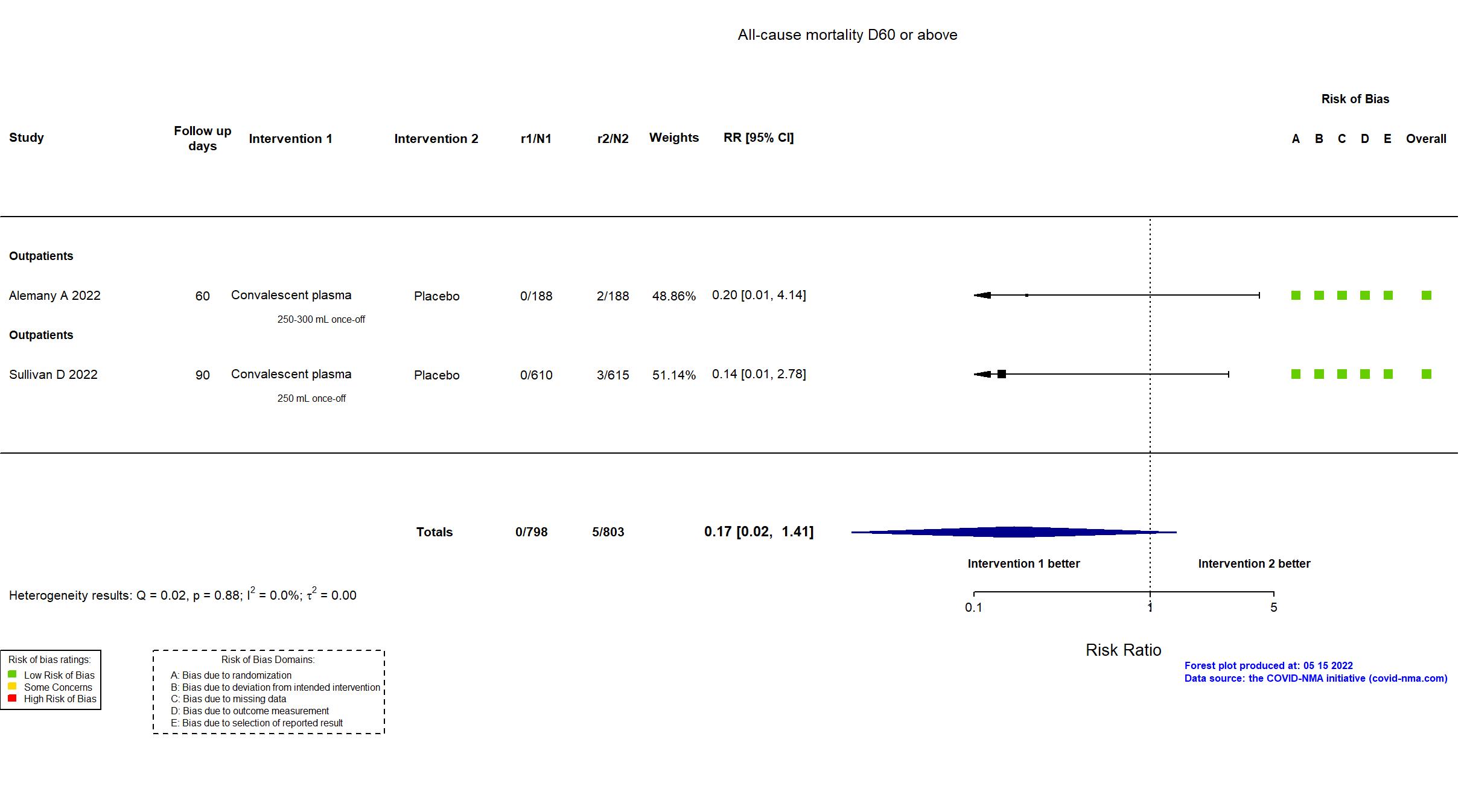
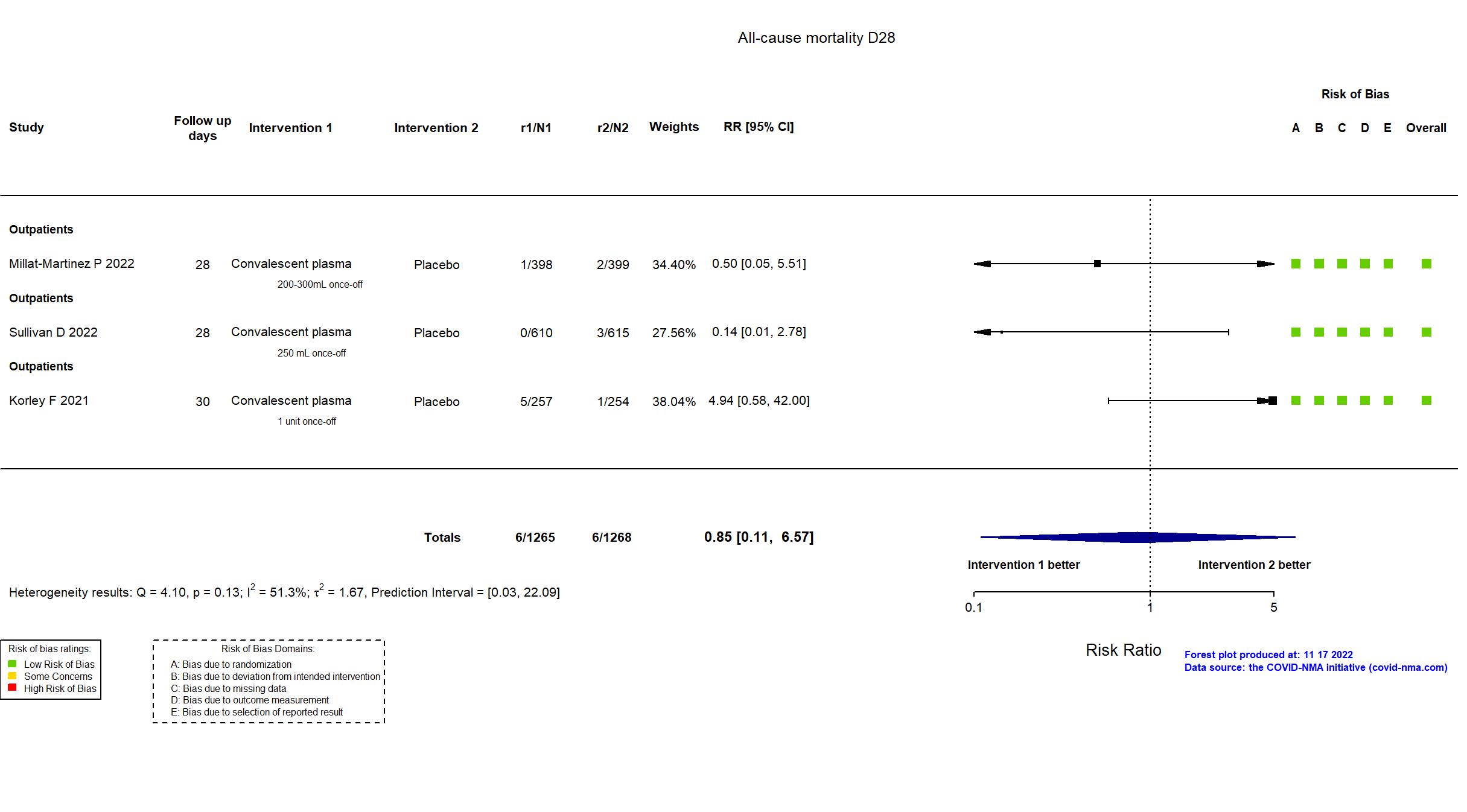
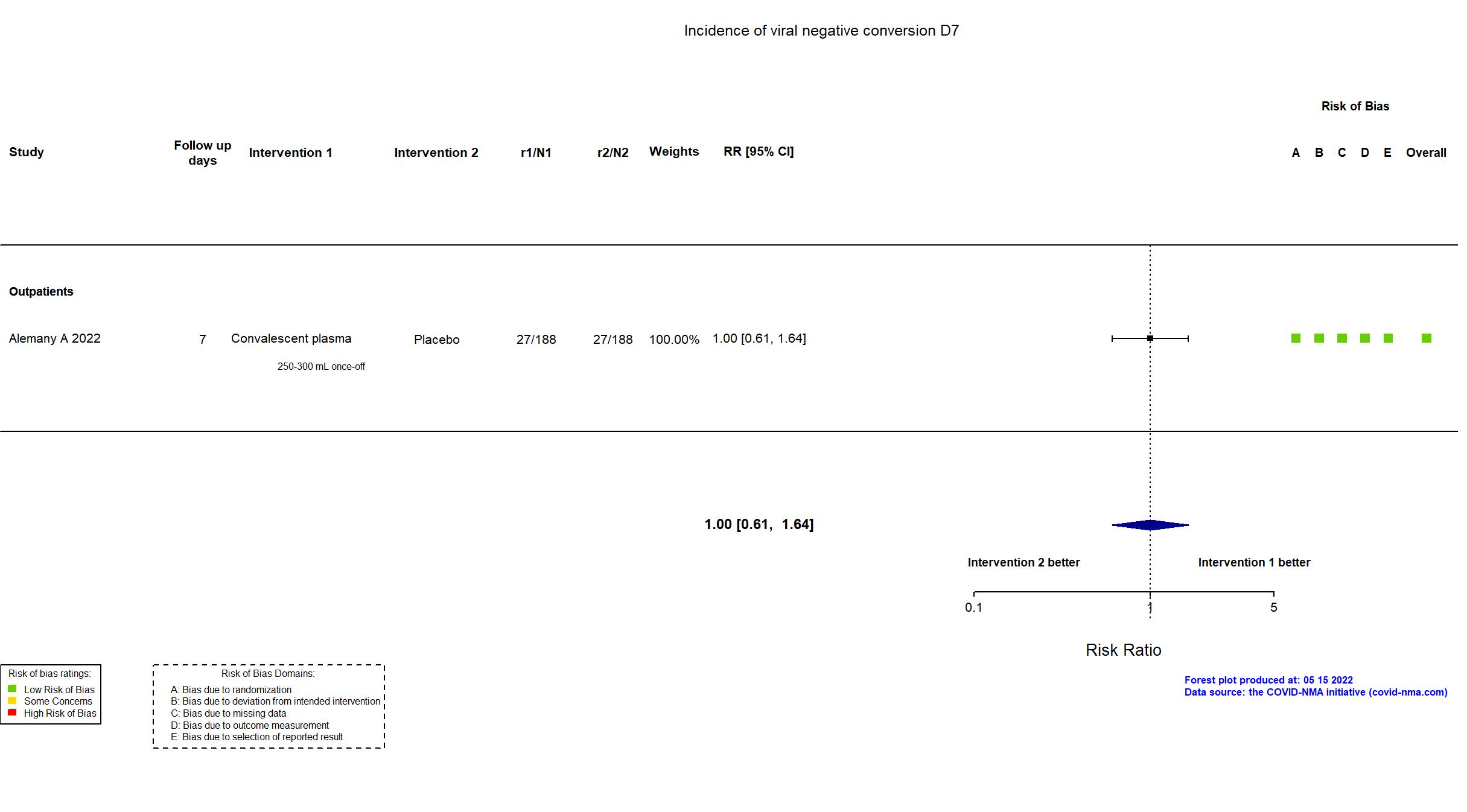
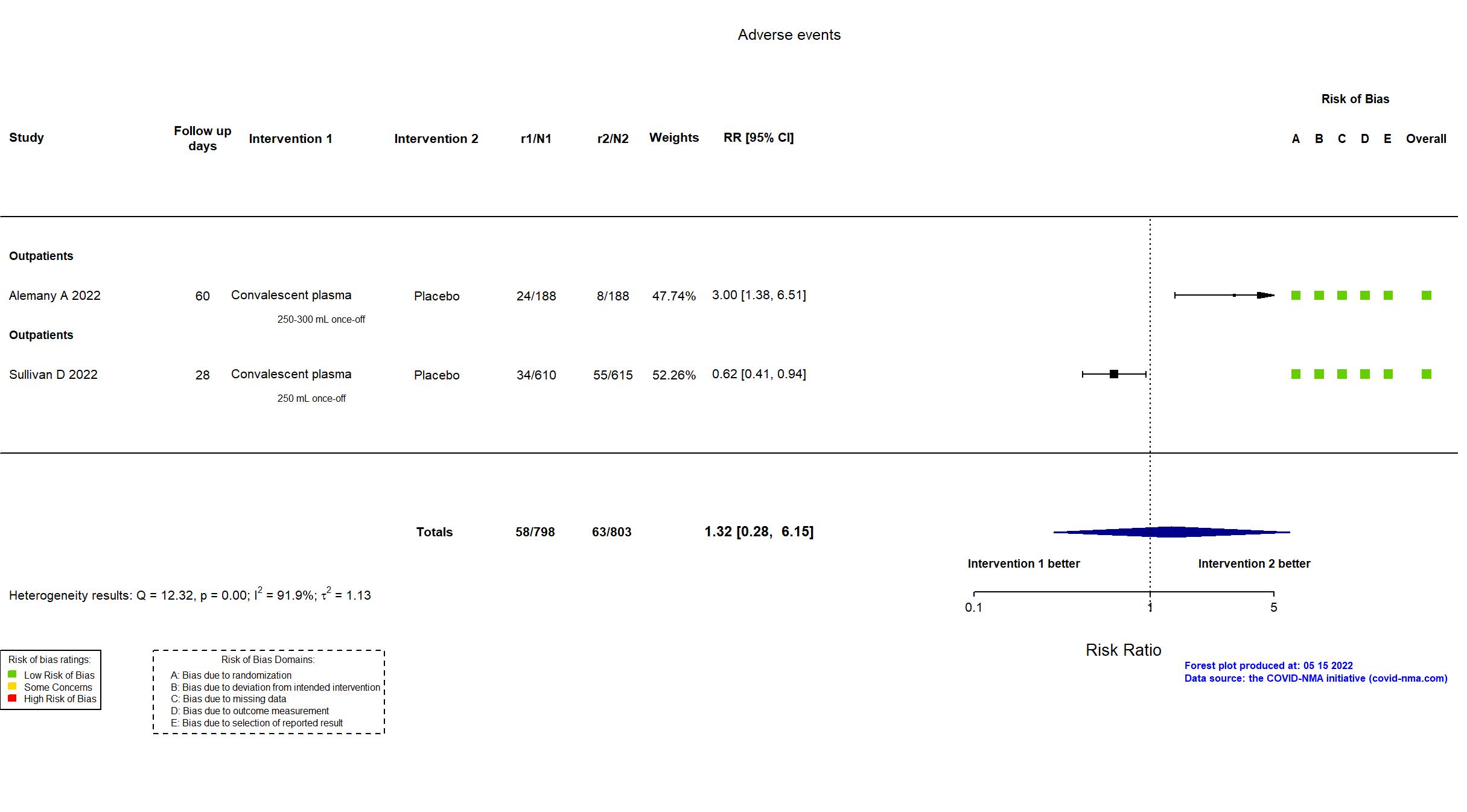
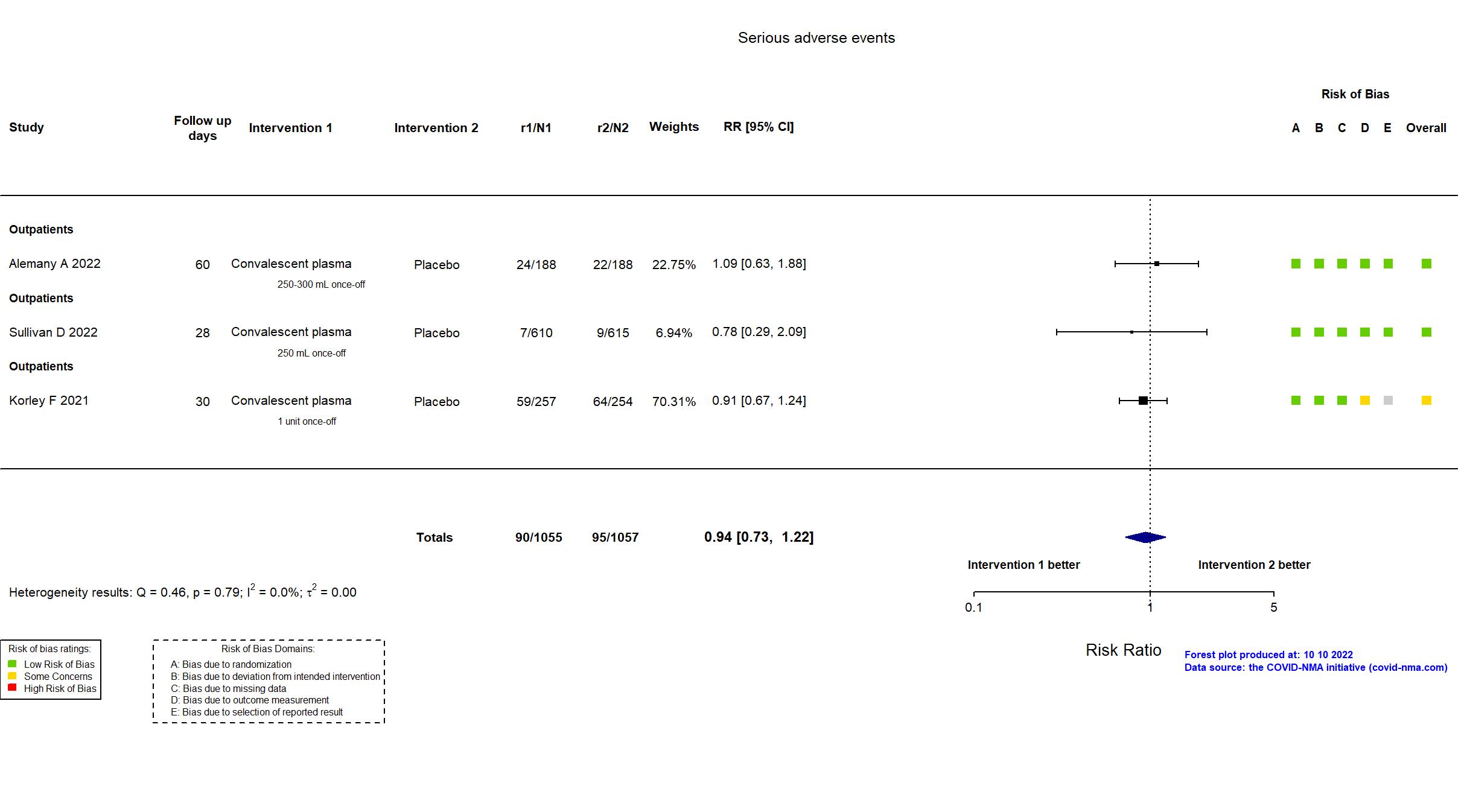
Studies description
Trial NCT04621123
Publication Alemany A, Lancet Respir Med (2022) (published paper)
Dates: 2020-11-10 to 2021-07-28
Funding: Mixed (The Fight AIDS and Infectious Diseases Foundation (Badalona, Spain) with funding from the pharmaceutical company, Grifols Worldwide Operations (Dublin, Ireland); the Crowdfunding campaign, YoMeCorono; the Hospital Universitari Germans Trias i Pujol, and Banc de Sang i Teixits de Catalunya.)
Conflict of interest: No
Trial NCT04355767
Publication SIREN-C3PO - Korley F, N Engl J Med (2021) (published paper)
Dates: 2020-08-11 to 2021-02-28
Funding: Public/non profit (National Heart, Lung, and Blood Institute and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health; the Biomedical Advanced Research and Development Authority; the Operation Warp Speed interagency program.)
Conflict of interest: Yes
Trial NCT04589949, NCT04621123
Publication COMPILE home - Millat-Martinez P, Nat Commun (2022) (published paper)
Dates: 2020-11-01 to 2021-07-13
Funding: Mixed (ZONMW (the Netherlands), SUPPORT-E, YoMeCorono, The Fight AIDS and Infectious Diseases Foundation with funding from the pharmaceutical company Grifols S.A.)
Conflict of interest: No
Trial NCT04373460
Publication Sullivan D, N Engl J Med (2022) (published paper)
Dates: 2020-06-03 to 2021-10-01
Funding: Mixed (U.S. Department of Defenses (DOD) Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND), in collaboration with the Defense Health Agency (DHA); Bloomberg Philanthropies; State of Maryland; the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases; NIH National Center for Advancing Translational Sciences; Division of Intramural Research NIAID NIH; Mental Wellness Foundation; Moriah Fund; Octapharma; HealthNetwork Foundation; the Shear Family Foundation.)
Conflict of interest: No








