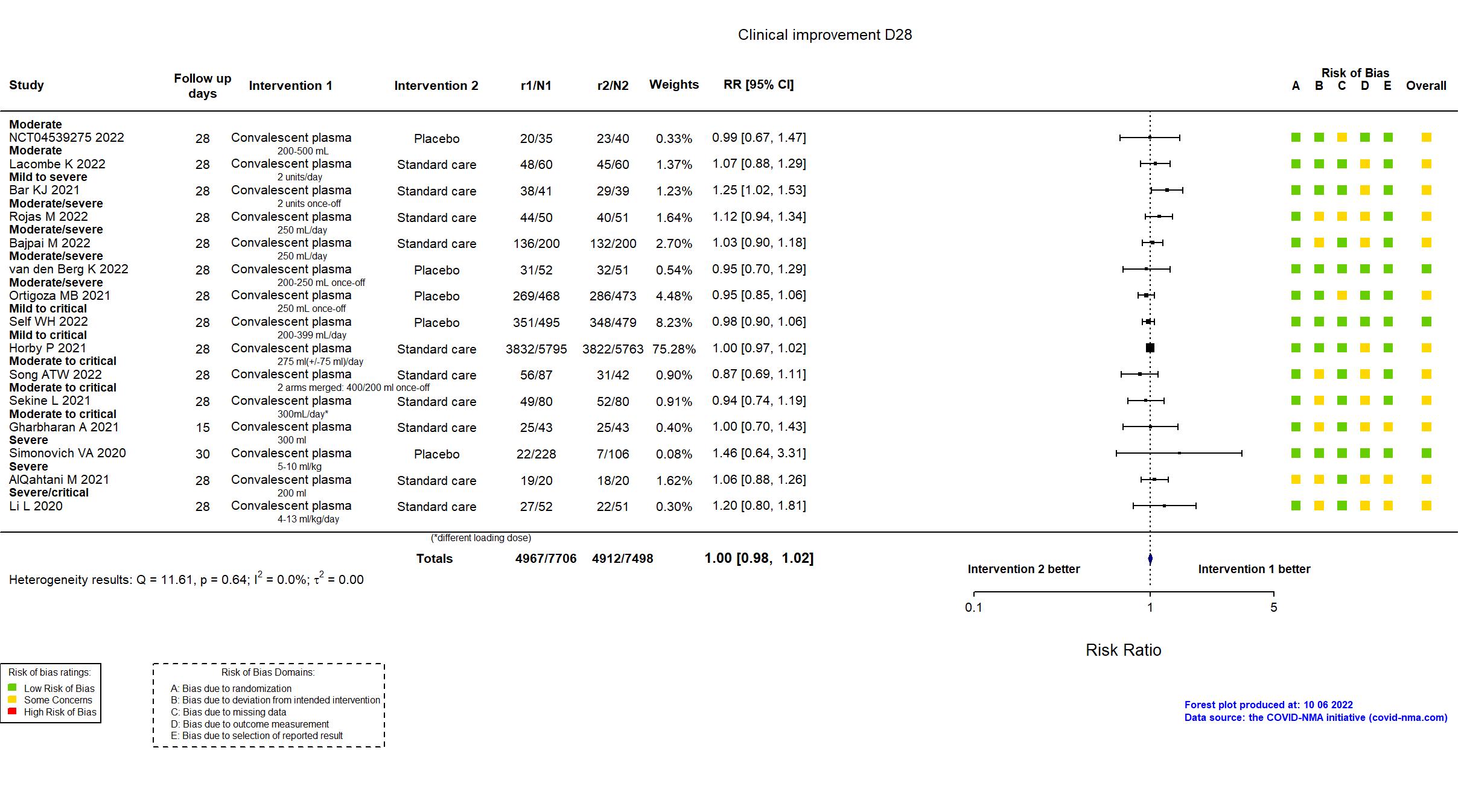
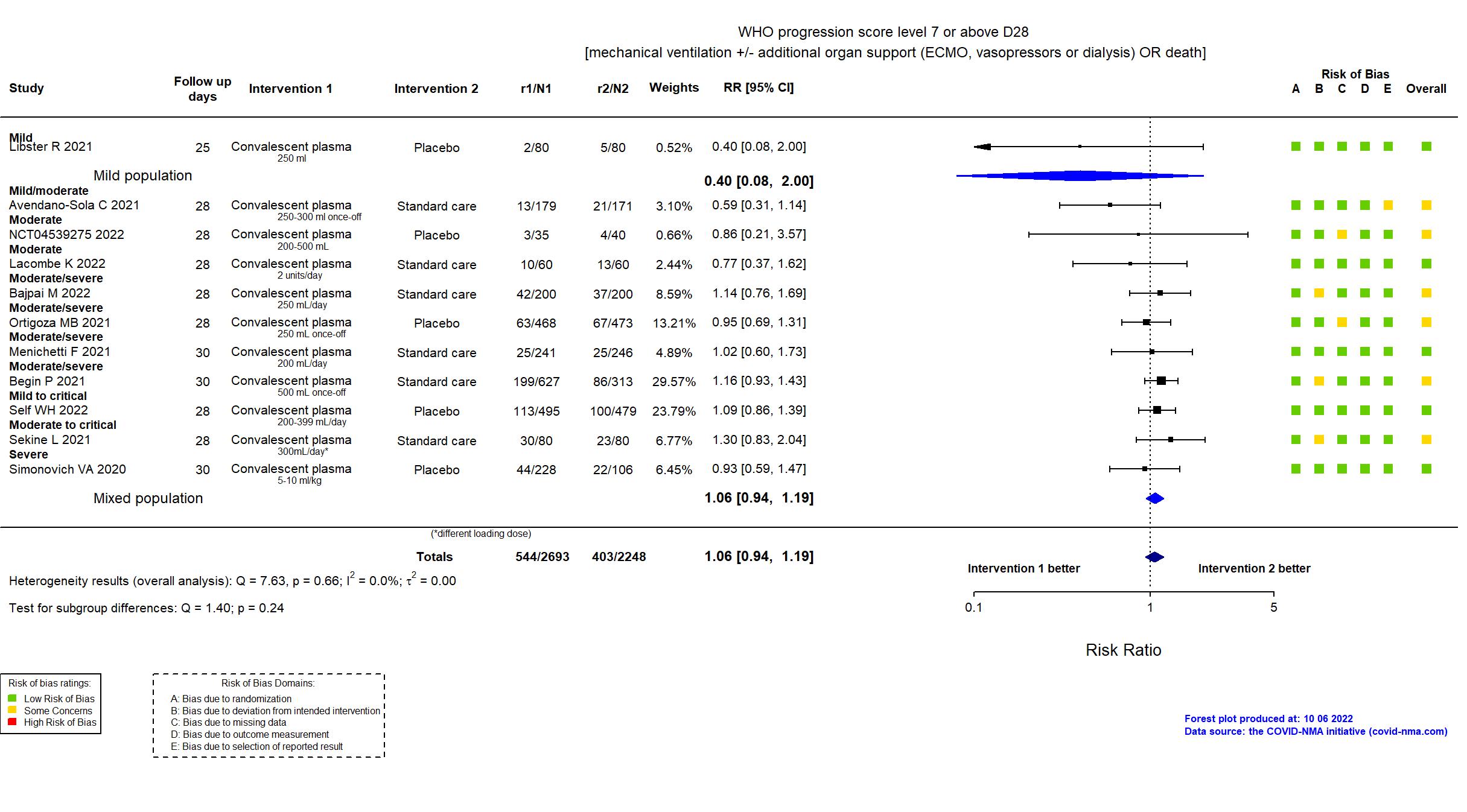
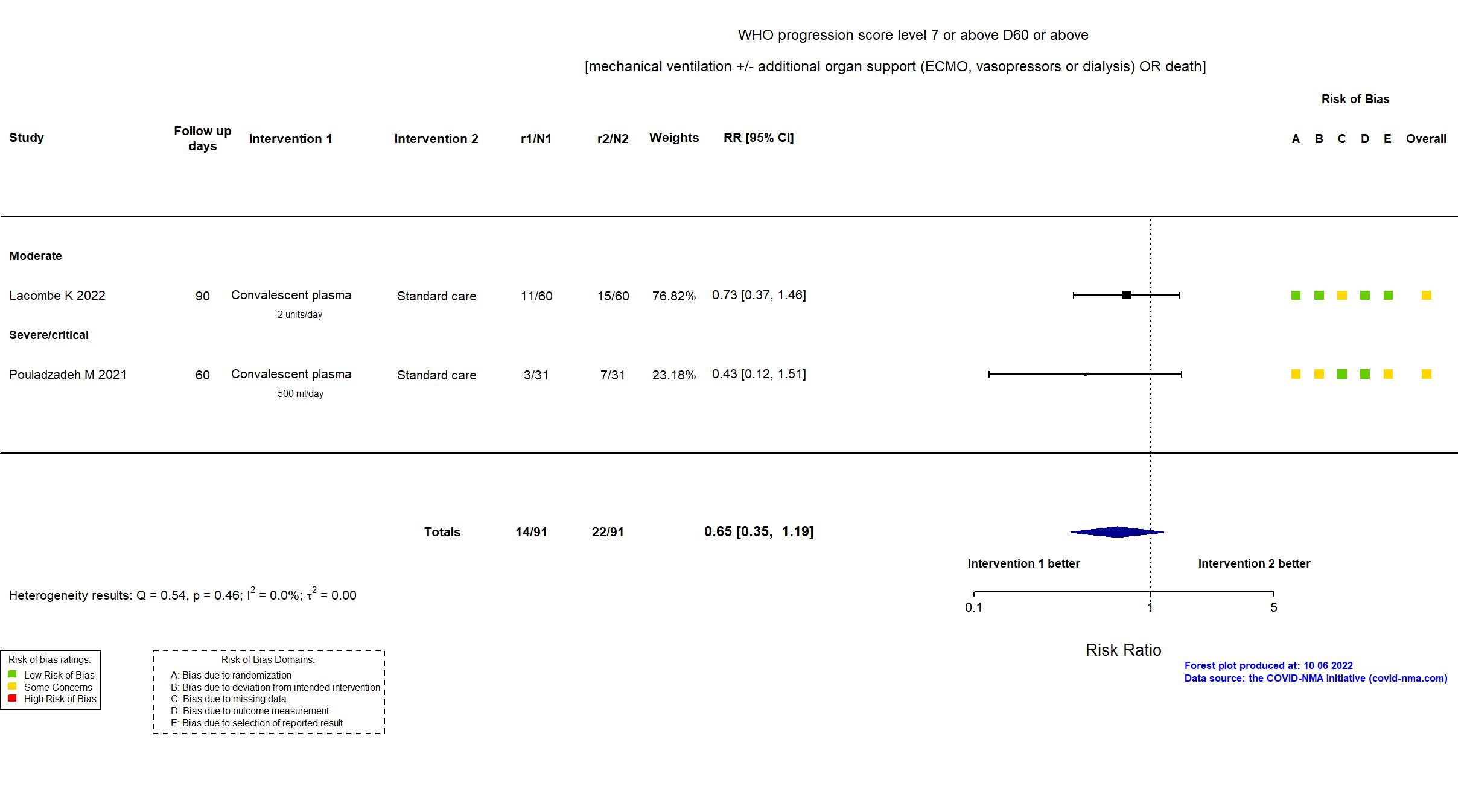
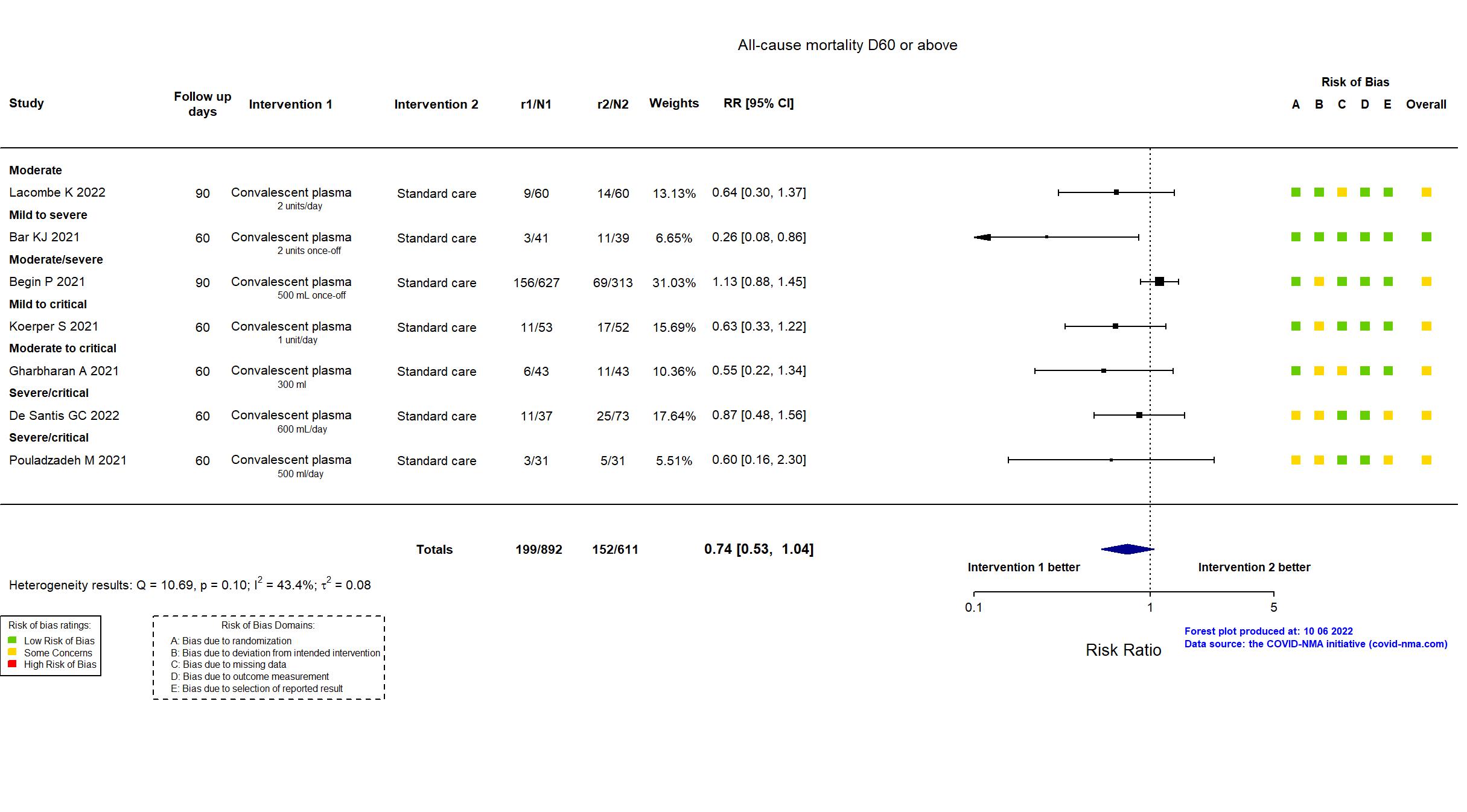
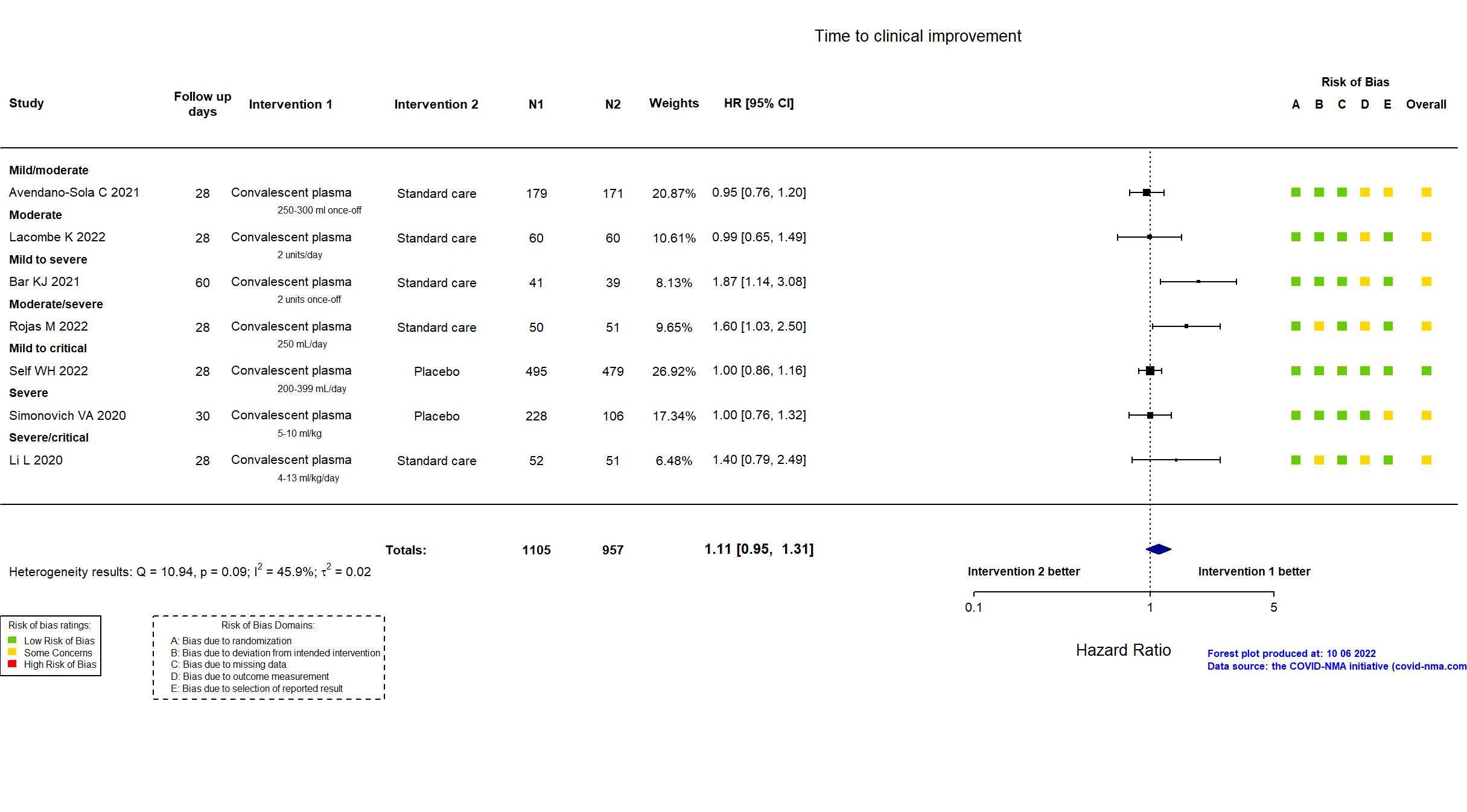
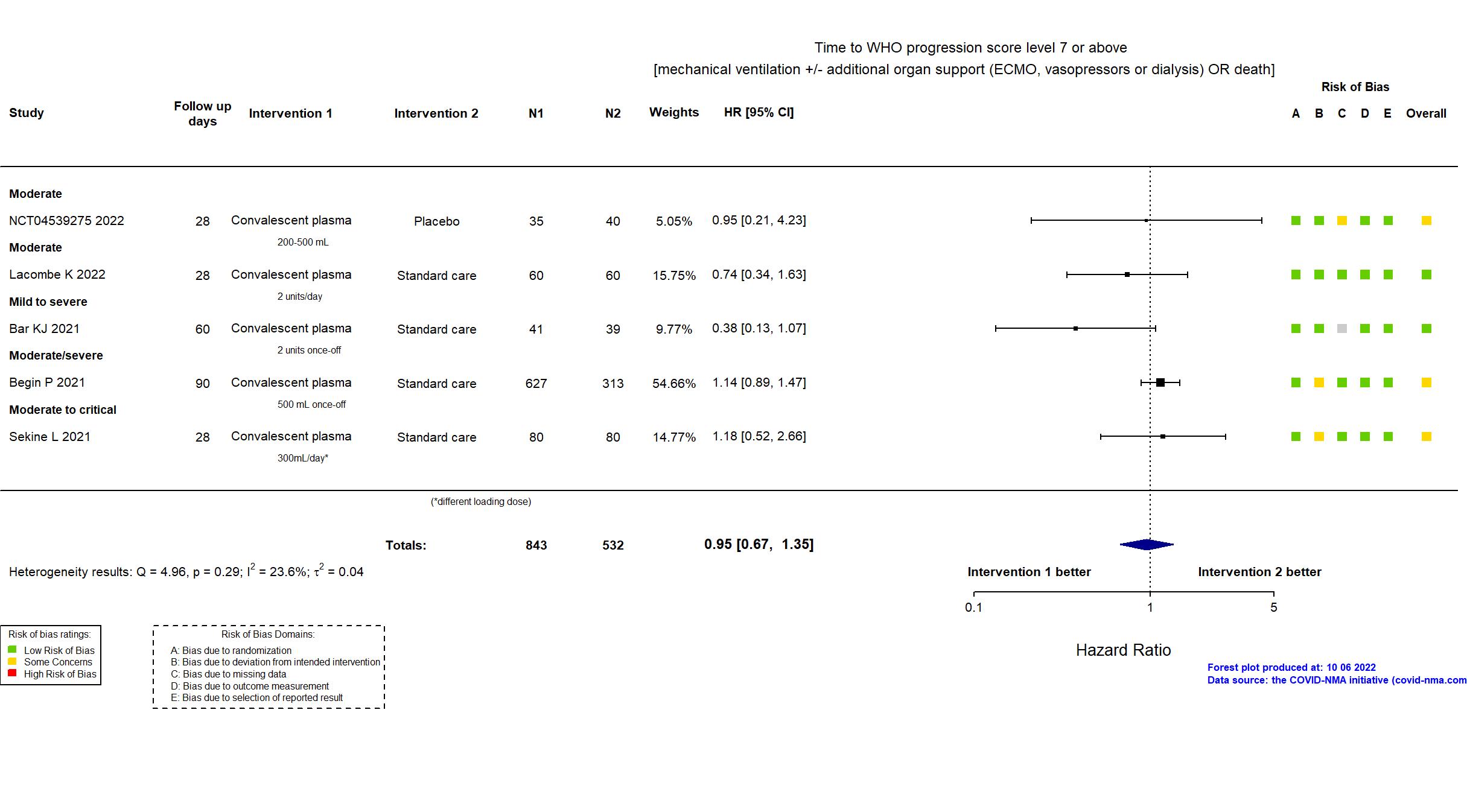
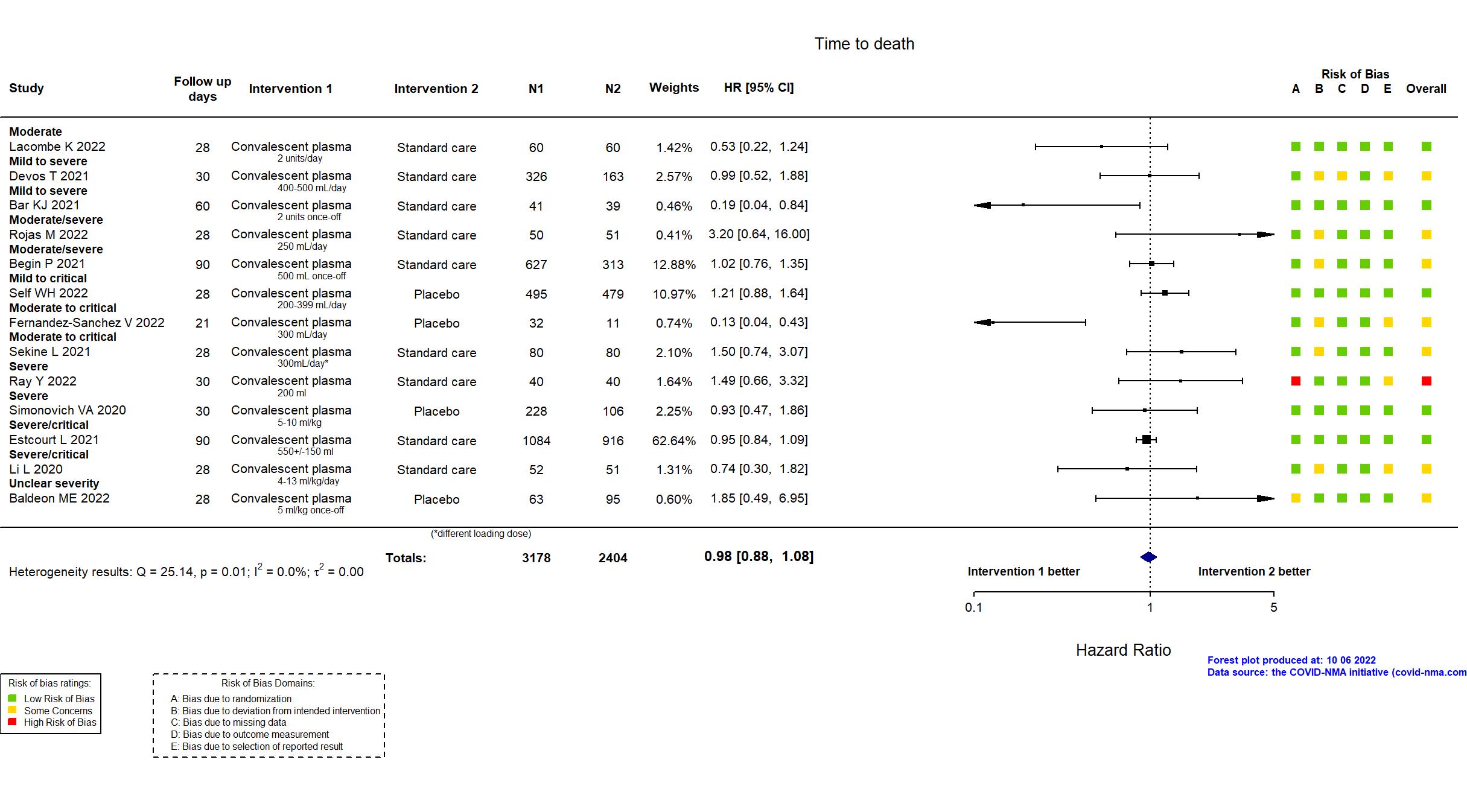
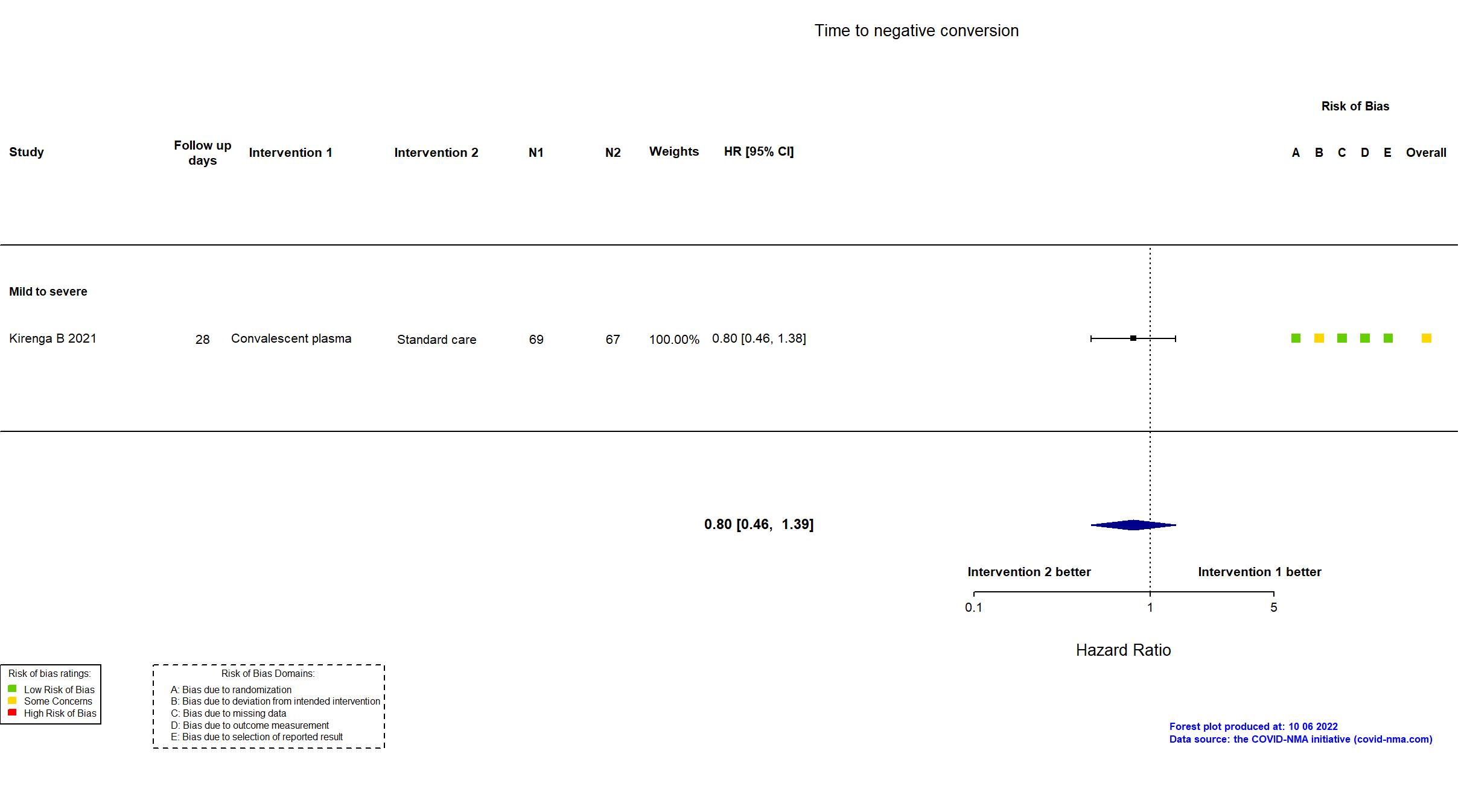
Convalescent plasma vs Standard care/Placebo (RCT)
Hospitalized patients
Studies included but not extracted/included in the analysis: Jalili E,Tanaffos, 2022; Denkinger C M, medrxiv, 2022 ; Thorlacius-Ussing L, Sci Rep, 2022;
Villanueva C, medrxiv, 2022
;
Muller-Tidow C, Hemasphere, 2022
FOREST PLOTS -2022-10-20

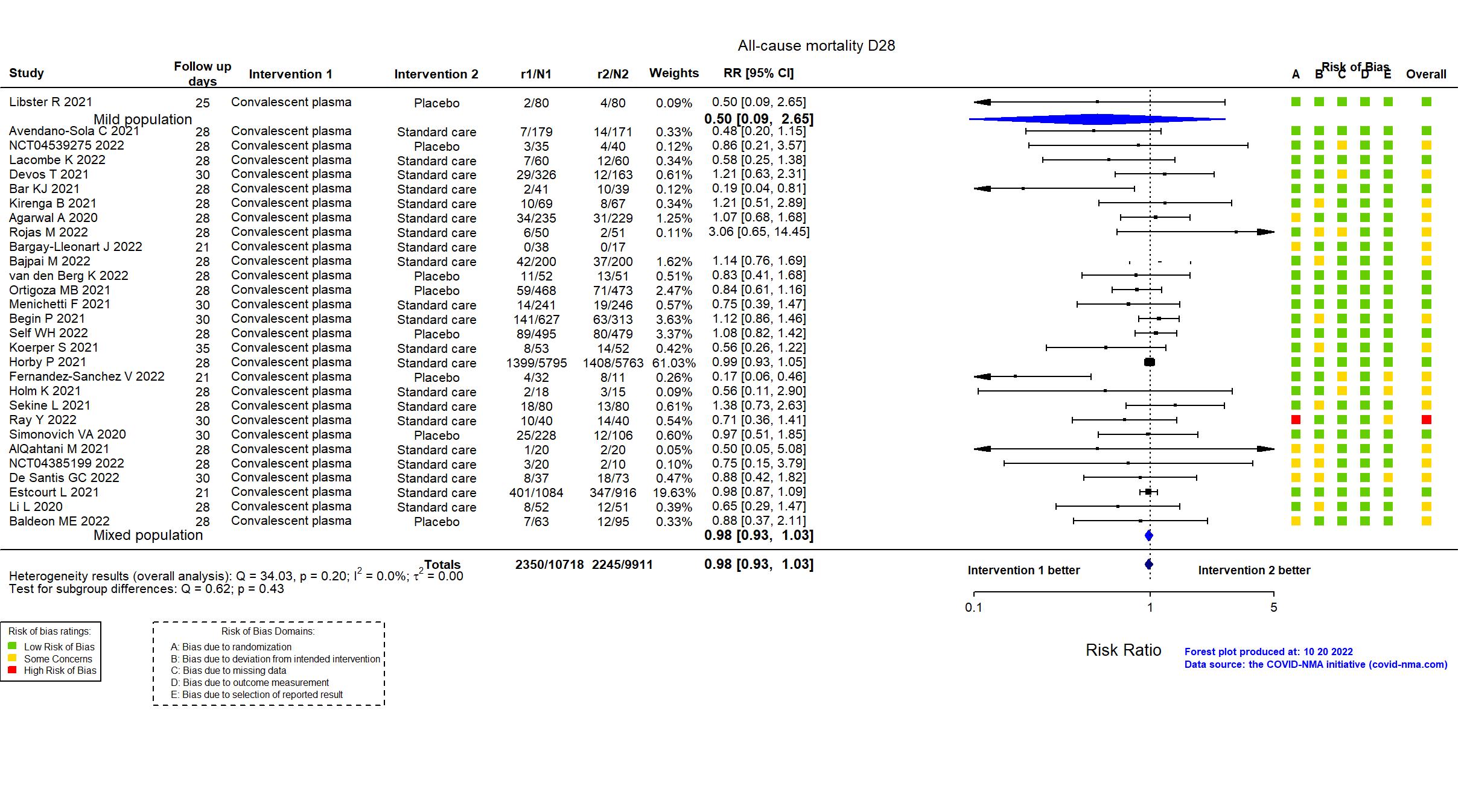
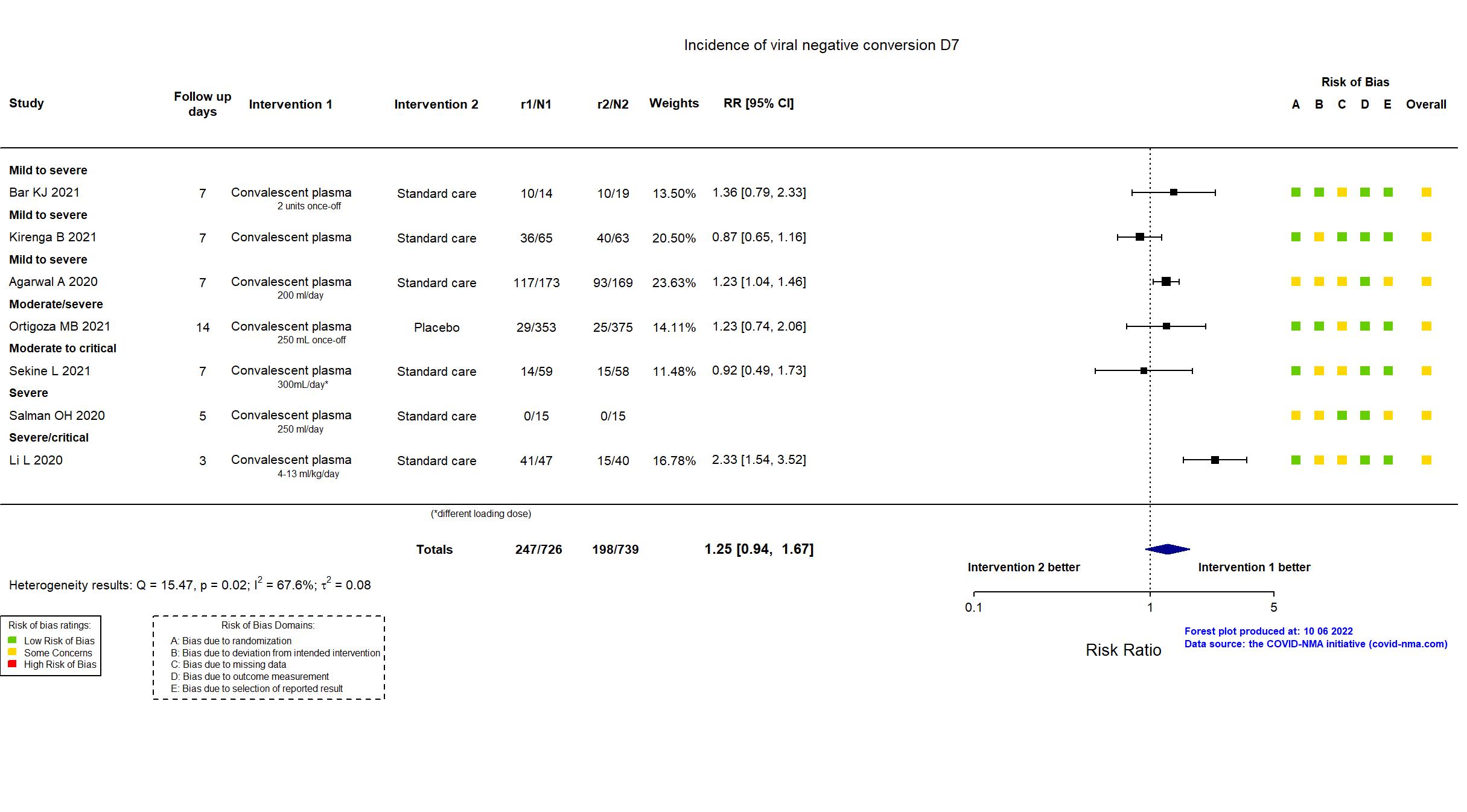
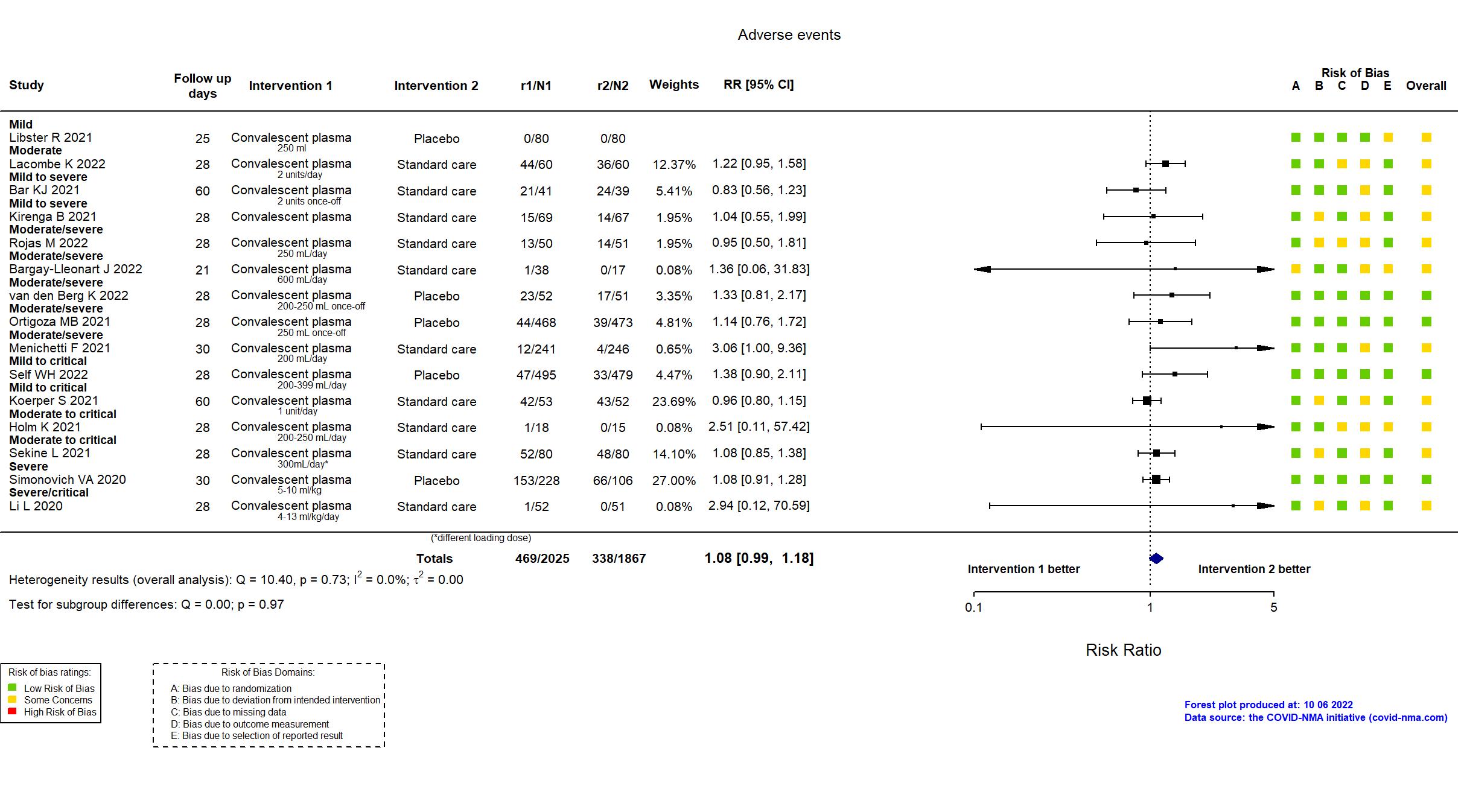
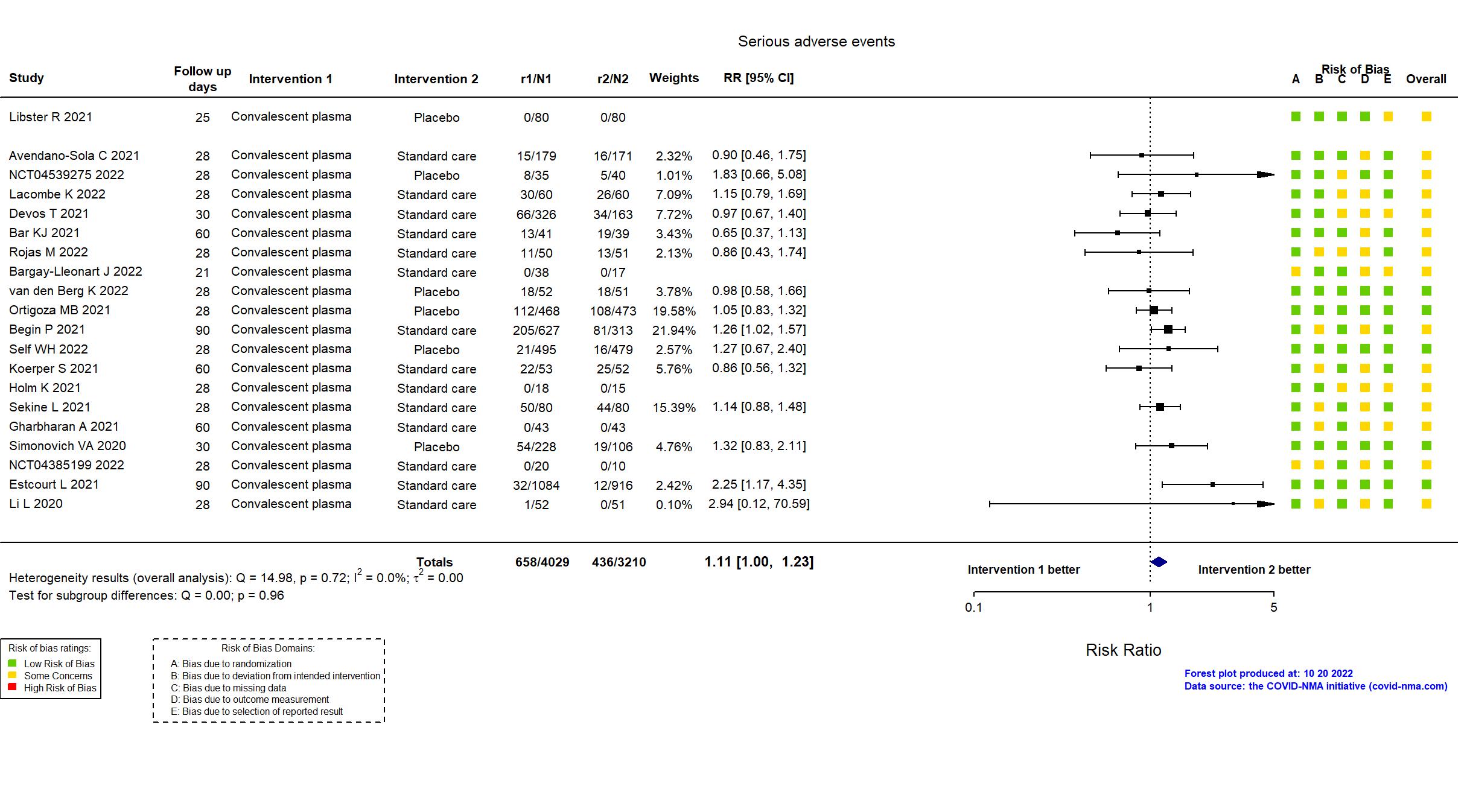
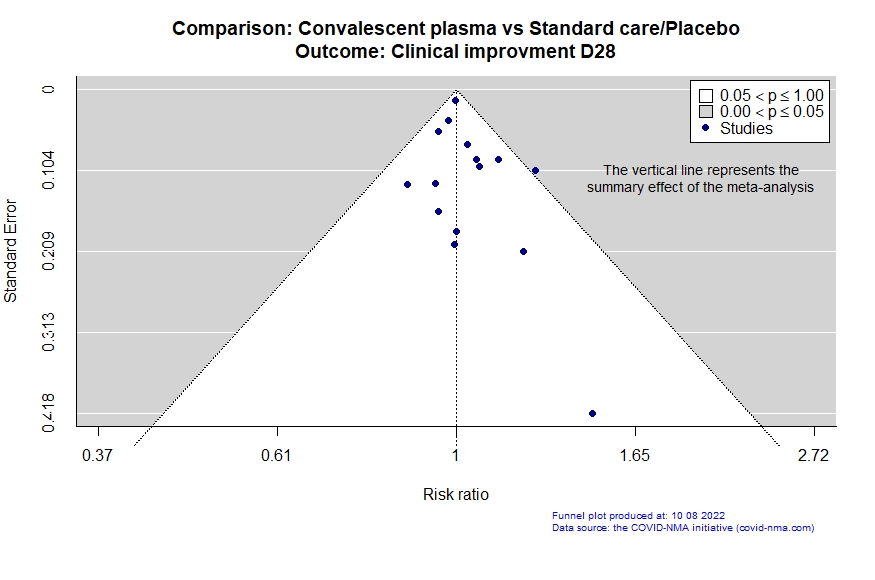
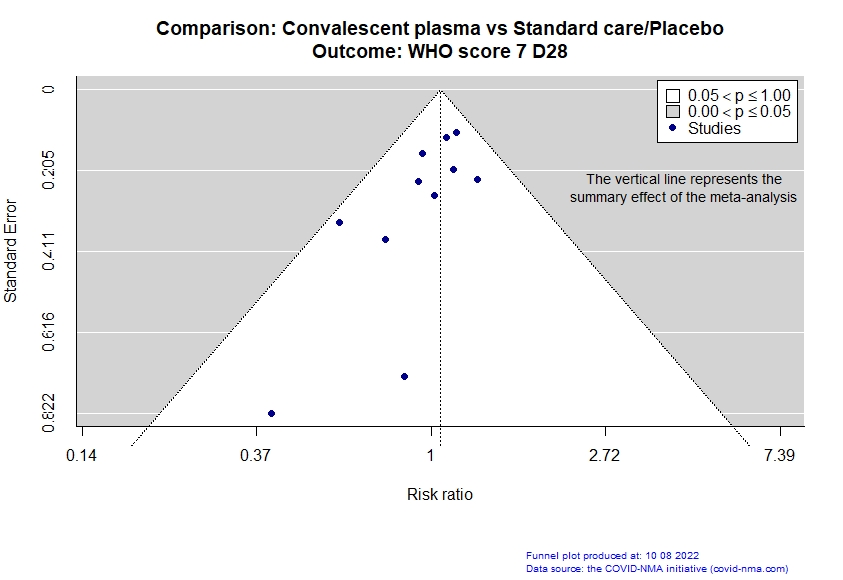
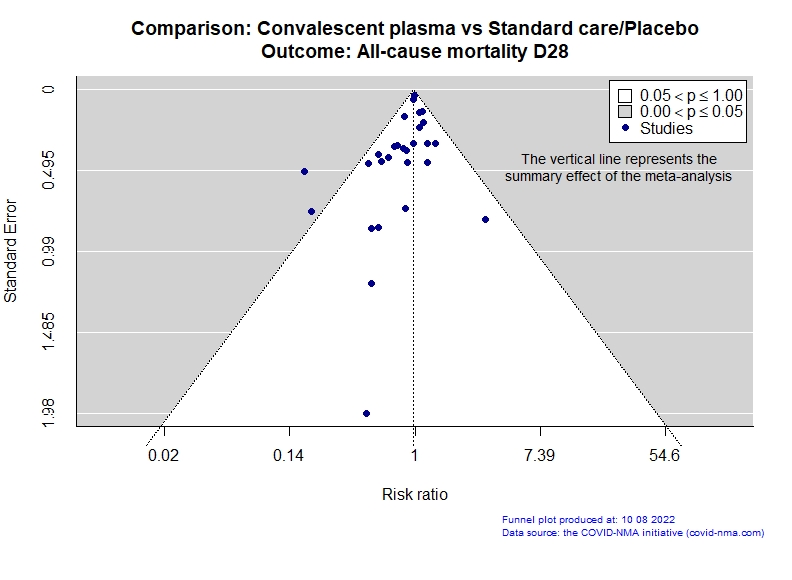
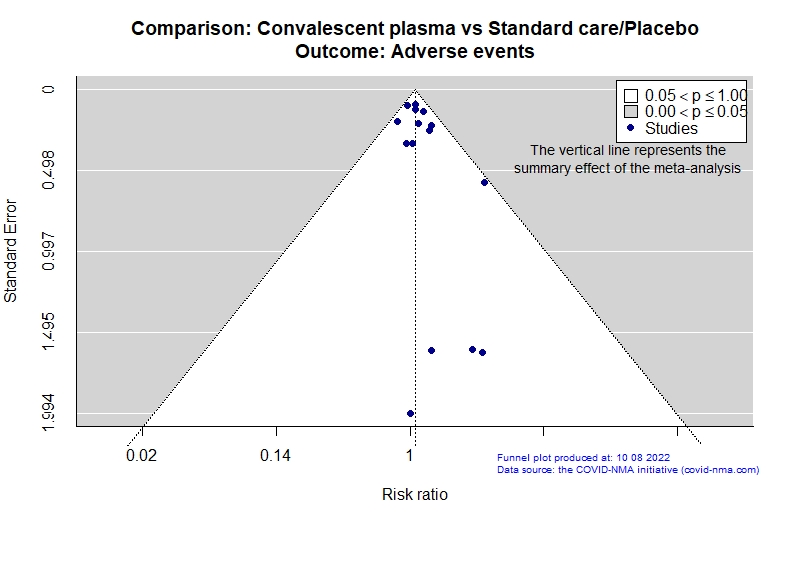
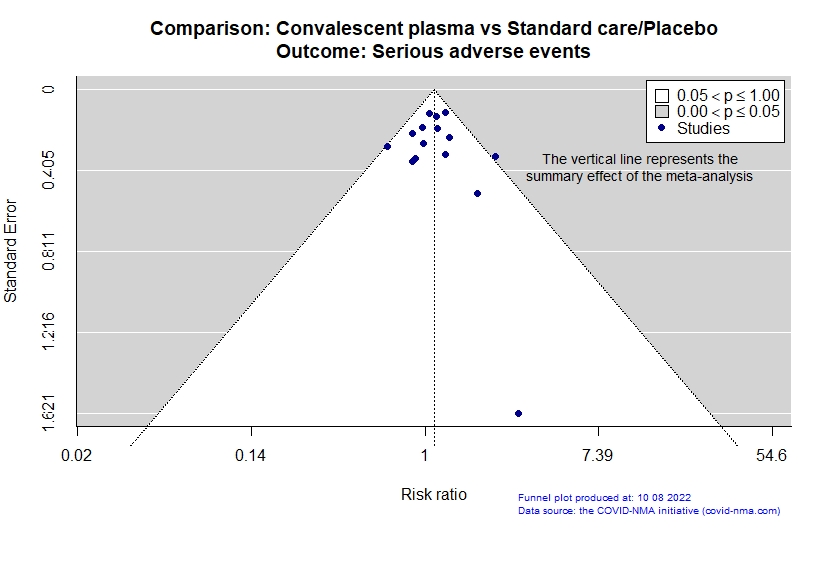
Studies description
Trial CTRI/2020/04/024775
Publication PLACID - Agarwal A, BMJ (2020) (published paper)
Dates: 2020-04-22 to 2020-07-14
Funding: Public/non profit (Indian Council of Medical Research (ICMR))
Conflict of interest: No
Trial NCT04356534
Publication AlQahtani M, Sci Rep (2021) (published paper)
Dates: 2020-04-19 to 2020-06-15
Funding: Public/non profit (Ministry of Health Bahrain and the College of Surgeons in Ireland-Bahrain)
Conflict of interest: No
Trial NCT04345523
Publication ConPlas-19 - Avendano-Sola C, J Clin Invest (2021) (published paper)
Dates: 2020-04-04 to 2021-02-05
Funding: Public/non profit (Government of Spain, Instituto de Salud Carlos III)
Conflict of interest: No
Trial NCT04425915
Publication COPLA-II trial - Bajpai M, BMJ Open (2022) (published paper)
Dates: 2020-06-14 to 2020-11-17
Funding: No specific funding (The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors. )
Conflict of interest: No
Trial ISRCTN85216856
Publication Baldeon ME, Transfus Med (2022) (published paper)
Dates: 2020-05-10 to 2020-10-31
Funding: Public/non profit (Salvar Vidas Ecuador (SalvarVidasEC))
Conflict of interest: No
Trial NCT04397757
Publication PennCCP2 - Bar KJ, J Clin Invest (2021) (published paper)
Dates: 2020-05-18 to 2021-01-08
Funding: Public/non profit (University of Pennsylvania. There was no commercial support for this trial.)
Conflict of interest: Yes
Trial NCT04803370
Publication Bargay-Lleonart J, J Clin Med (2022) (published paper)
Funding: Public/non profit (Health Research Institute of the Balearic Islands)
Conflict of interest: No
Trial NCT04348656
Publication CONCOR-1 - Begin P, Nat Med (2021) (published paper)
Dates: 2020-05-14 to 2021-01-29
Funding: Public/non profit (Canadian Institutes of Health Research; Ontario COVID-19 Rapid Research Fund; Toronto COVID-19 Action Initiative 2020 (University of Toronto); University Health Network Emergent Access Innovation Fund; University Health Academic Health Science Centre Alternative Funding Plan (Sunnybrook Health Sciences Centre); Ministère de l'Économie et de l'Innovation (Québec); Fond de Recherche du Québec en Santé; Saskatchewan Ministry of Health; University of Alberta Hospital Foundation; Alberta Health Services COVID-19 Foundation Competition;
Sunnybrook Health Sciences Centre Foundation; Fondations CHU Ste-Justine; The Ottawa Hospital Academic Medical Organization; The Ottawa Hospital Foundation COVID-19 Research Fund; Fondation du CHUM; Sinai Health System Foundation and McMaster University. These did not have any role in the writing of the manuscript or the decision to submit it for publication. The authors did not receive payments from any pharmaceutical company or other agency to write this article.)
Conflict of interest: No
Trial RBR-7f4mt9f
Publication De Santis GC, Emerg Infect Dis (2022) (published paper)
Dates: 2020-04-14 to 2020-11-30
Funding: Public/non profit (Fundação de Amparo à Pesquisa do Estado de São Paulo)
Conflict of interest: *
Trial NCT04429854
Publication DAWN-plasma trial - Devos T, Eur Respir J (2021) (published paper)
Dates: 2020-05-02 to 2021-01-26
Funding: Public/non profit (Fonds Wetenschappelijk Onderzoek (FWO) [Research Foundation – Flanders]; Belgian Health Care Knowledge Centre (KCE))
Conflict of interest: No
Trial NCT02735707
Publication REMAP-CAP - Estcourt L, JAMA (2021) (published paper)
Dates: 2020-05-05 to 2021-01-18
Funding: Mixed (PREPARE consortium by the European Union; Australian National Health and Medical Research Council; Australian Medical Research Future Fund; New Zealand Health Research Council; Canadian Institutes of Health Research COVID-19 Rapid Research Funding; Canadian Institute of Health Research Strategy for Patient-Oriented Research Innovative Clinical Trials Program Grant; NIHR; UK NIHR; NIHR Imperial Biomedical Research Centre; Health Research Board of Ireland; UPMC Learning While Doing Program; Translational Breast Cancer Research Consortium; Pittsburgh Foundation; French Ministry of Health; Minderoo Foundation; Wellcome Trust Innovations Project; Australian government; DHSC; EU SoHo Grants)
Conflict of interest: Yes
Trial NCT04405310
Publication Fernandez-Sanchez V, Research Square (2022) (preprint)
Dates: 2020-05-20 to 2020-10-10
Funding: Public/non profit (CEMEVAV (Centro Medico Naval, Naval Medical Center))
Conflict of interest: No
Trial NCT04342182
Publication Gharbharan A, Nat Commun (2021) (published paper)
Dates: 2020-04-08 to 2020-06-14
Funding: Mixed (The Erasmusfoundation, Ypsilo and Health Holland)
Conflict of interest: No
Trial NCT04600440
Publication Holm K, BMC Res Notes (2021) (published paper)
Dates: 2020-06-01 to 2021-01-30
Funding: Public/non profit (Skåne Region)
Conflict of interest: No
Trial NCT04381936; EudraCT 2020-001113-21; ISRCTN5018967
Publication RECOVERY-CP - Horby P, Lancet (2021) (published paper)
Dates: 2020-05-28 to 2021-01-15
Funding: Public/non profit (UK Research and Innovation (Medical Research Council) and National Institute of Health Research )
Conflict of interest: No
Trial NCT04542941
Publication Kirenga B, BMJ Open Respir Res (2021) (published paper)
Dates: 2020-09-23 to 2020-12-02
Funding: Public/non profit (Government of Uganda through the Makerere University Research and Innovations Fund)
Conflict of interest: No
Trial NCT04433910; EudraCT 2020-001310-38
Publication CAPSID - Koerper S, J Clin Invest (2021) (published paper)
Dates: 2020-08-30 to 2020-12-24
Funding: Public/non profit (Bundesministerium fuer Gesundheit (German Federal Ministry of Health))
Conflict of interest: Yes
Trial NCT04345991
Publication CORIPLASM - Lacombe K, Vox Sang (2022) (unpublished results)
Dates: 2020-04-16 to 2021-04-21
Funding: Public/non profit (Programme Hospitalier de Recherche Clinique ; Fondation pour la Recherche Médicale ; Sorbonne Université Paris )
Conflict of interest: *
Trial ChiCTR2000029757
Publication Li L, JAMA (2020) (published paper)
Dates:

















