Proxalutamide vs Standard care/Placebo (RCT)
Hospitalized patients
Study McCoy J, Frontiers (2021) has been retracted on June 10, 2022. The study was excluded from the analysis and grade assessment by August 10 the latest.
FOREST PLOTS -2021-07-08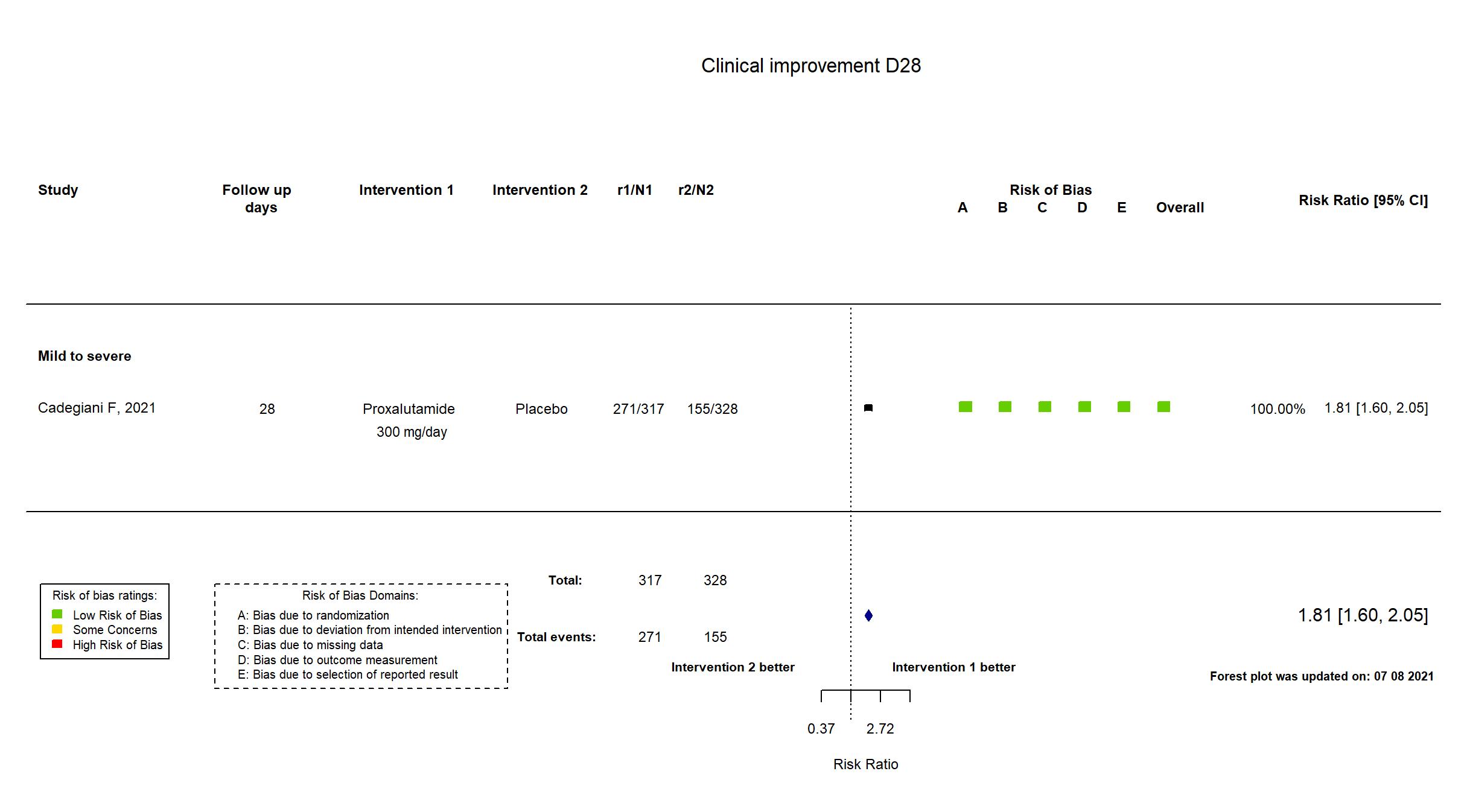
Study McCoy J, Frontiers (2021) has been retracted on June 10, 2022. The study was excluded from the analysis and grade assessment by August 10 the latest.
FOREST PLOTS -2021-07-08

Trial NCT04728802
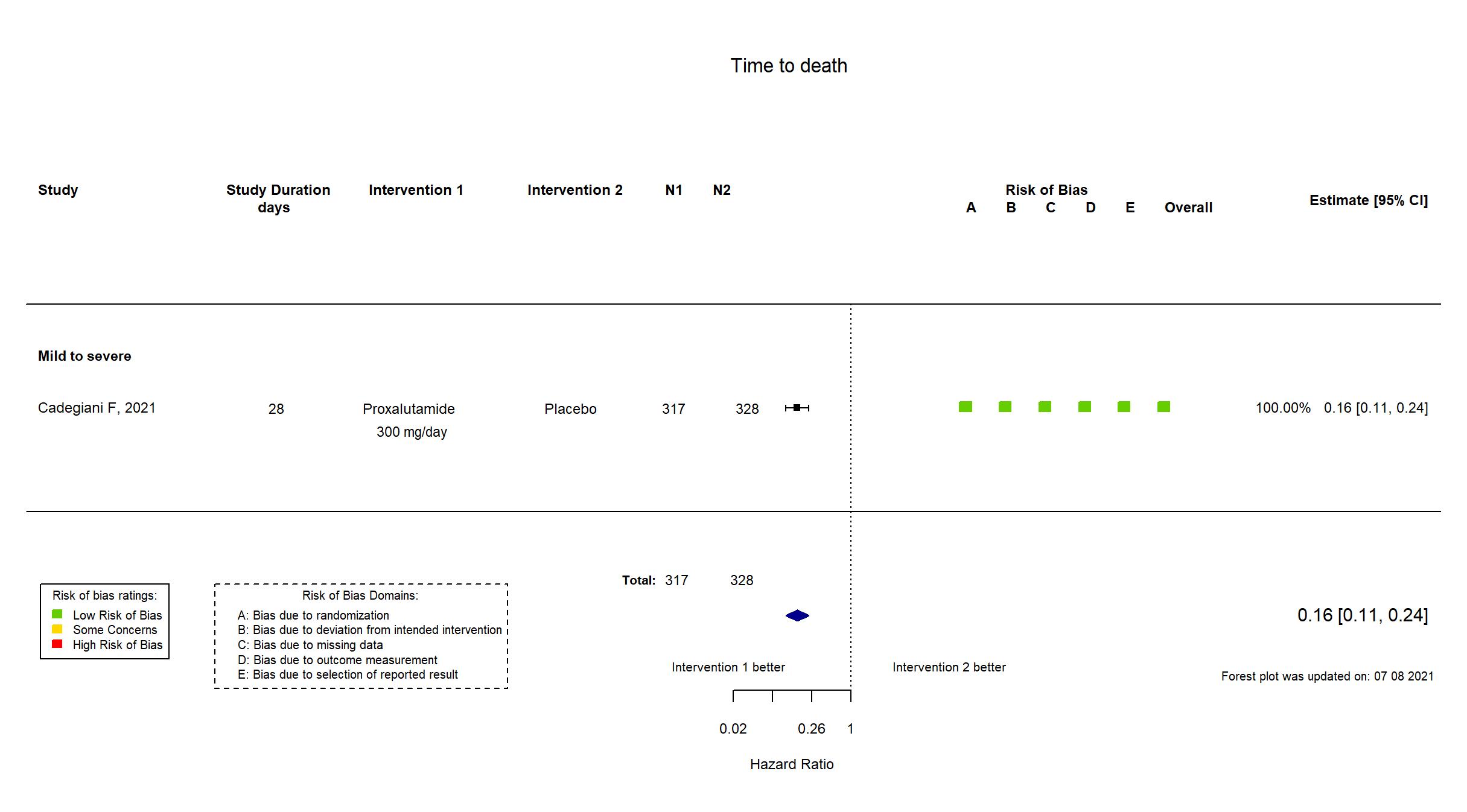
Publication Cadegiani F, medRxiv (2021) (preprint)
Dates: 2021-02-01 to 2021-03-17
Funding: Private (Kintor Pharmaceuticals, Ltd)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / Brazil Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Proxalutamide 300 mg/day orally for 14 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Proxalutamide =317 Placebo=328 | |
| Characteristics of participants N= 645 Mean age : NR 366 males Severity : Mild: n=20 / Moderate: n=196 / Severe: n=429 Critical: n=0 | |
| Primary outcome | |
| In the register 14 Day Recovery Rate [ Time Frame: Day 14 ] Recovery rate defined as those reaching scores 1 or 2 over 14 days after randomization on the COVID-19 ordinal scale. The ordinal scale is defined as follows: 8. Death; 7. Hospitalized, on invasive mechanical ventilation or ECMO; 6. Hospitalized, on non-invasive ventilation or high flow oxygen devices; 5. Hospitalized, requiring supplemental oxygen; 4. Hospitalized, not requiring supplemental oxygen- requiring ongoing medical care (COVID-19 related or otherwise; 3. Hospitalized, not requiring supplemental oxygen - no longer requires ongoing medical care; 2. Not hospitalized, limitation on activities; 1. Not hospitalized, no limitations on activities | |
| In the report Clinical status (8-point ordinal scale) at 14-days post-randomization: 14-day recovery ratio (alive hospital discharge [scores 1, 2]). | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment | In addition to the pre-print article, the prospective trial registry, protocol, statistical analysis plan and supplementary materials were used in data extraction and assessment of risk of bias. There were some minor differences between secondary endpoints in the pre-print article and in the registry and protocol. The trial (n = 645) achieved its target sample size (n = 600). |