Umifenovir vs Standard care (RCT)
Hospitalized patients
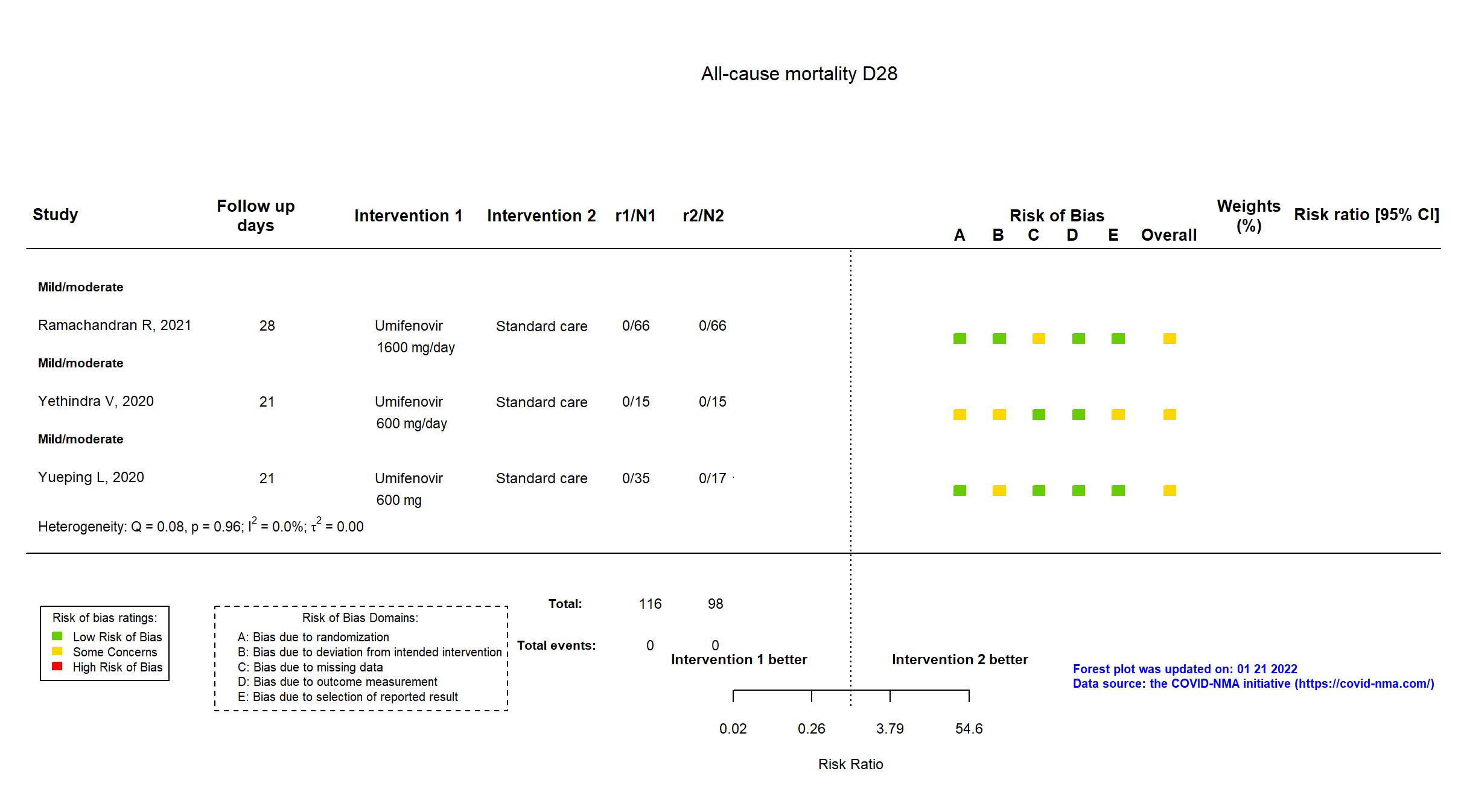
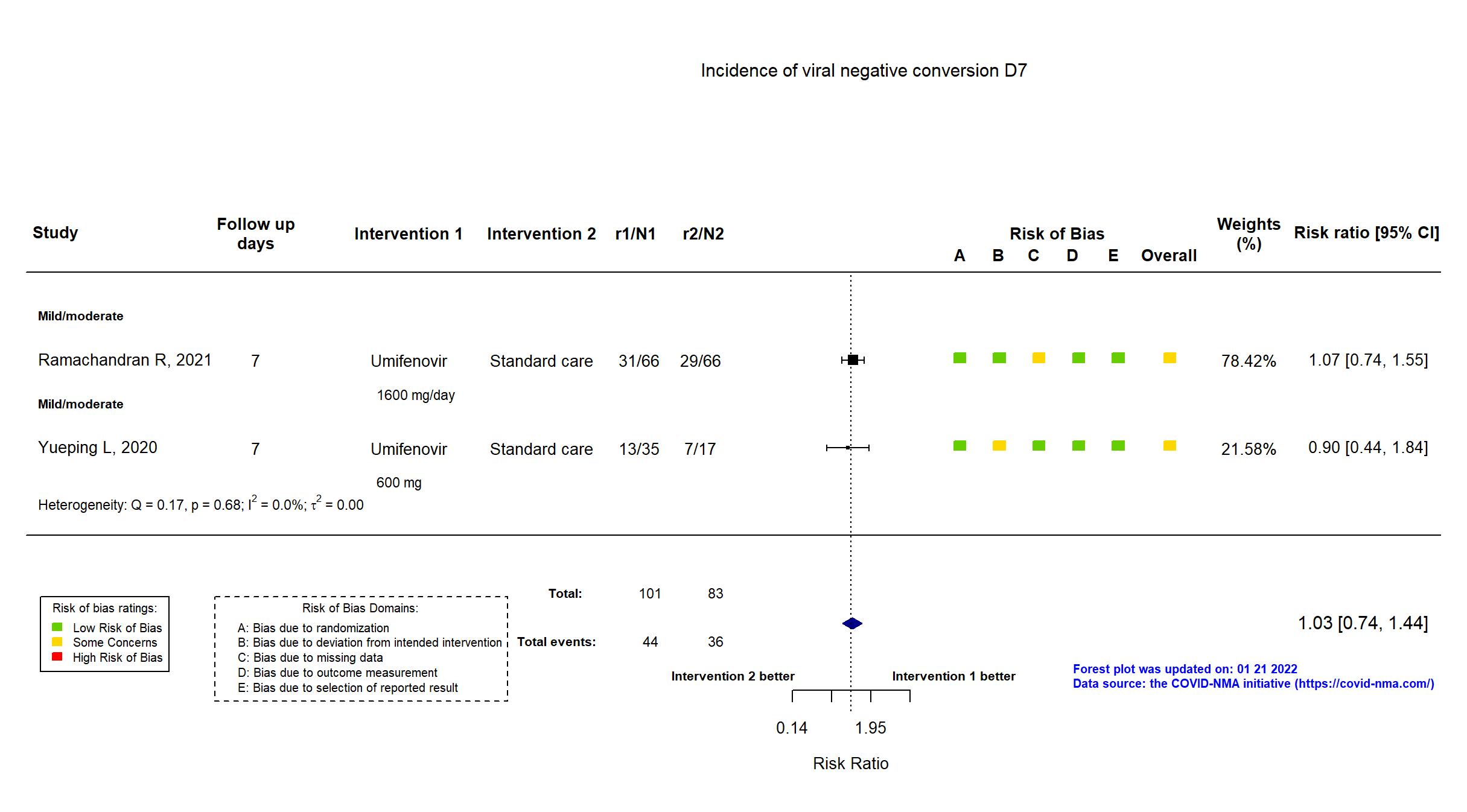
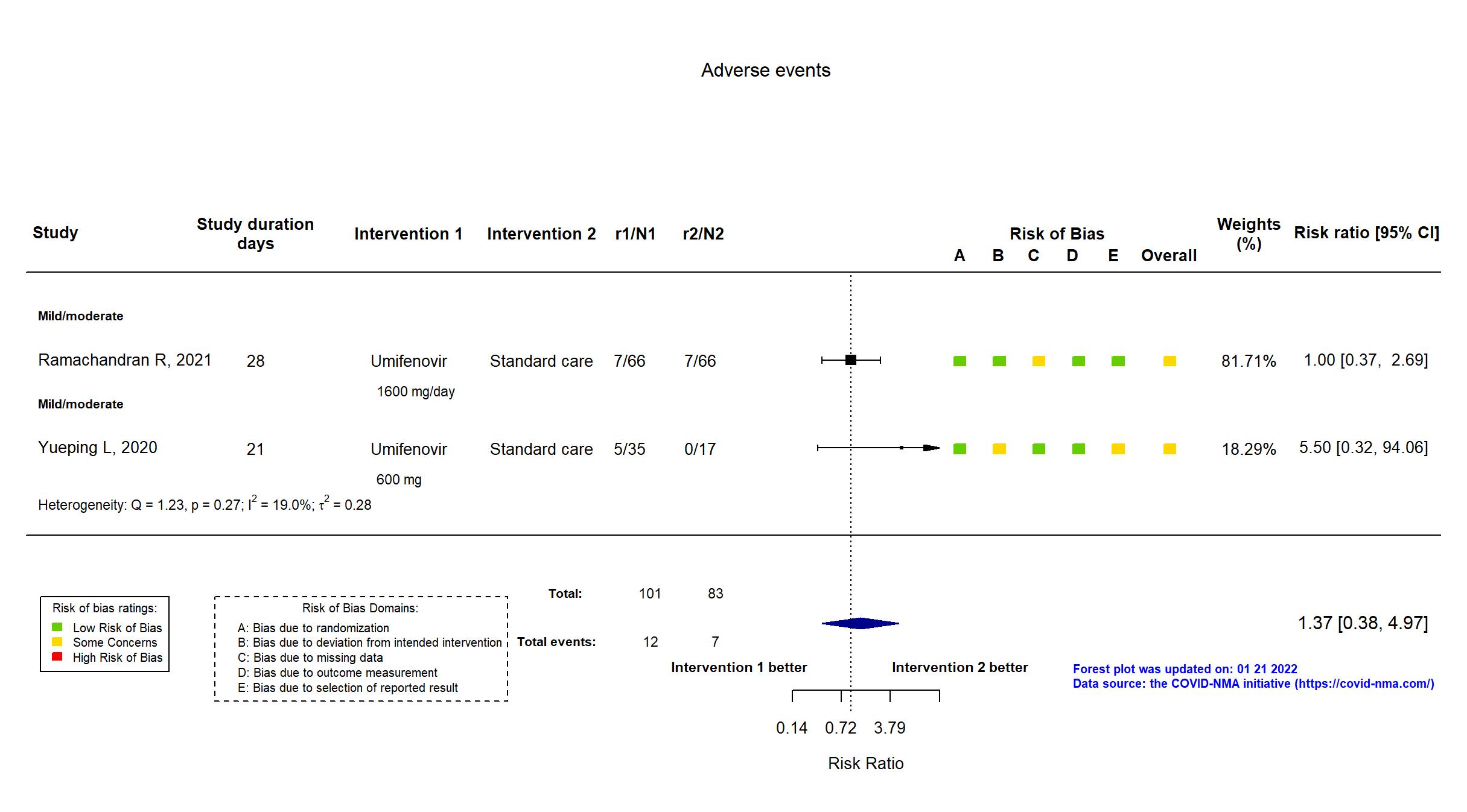
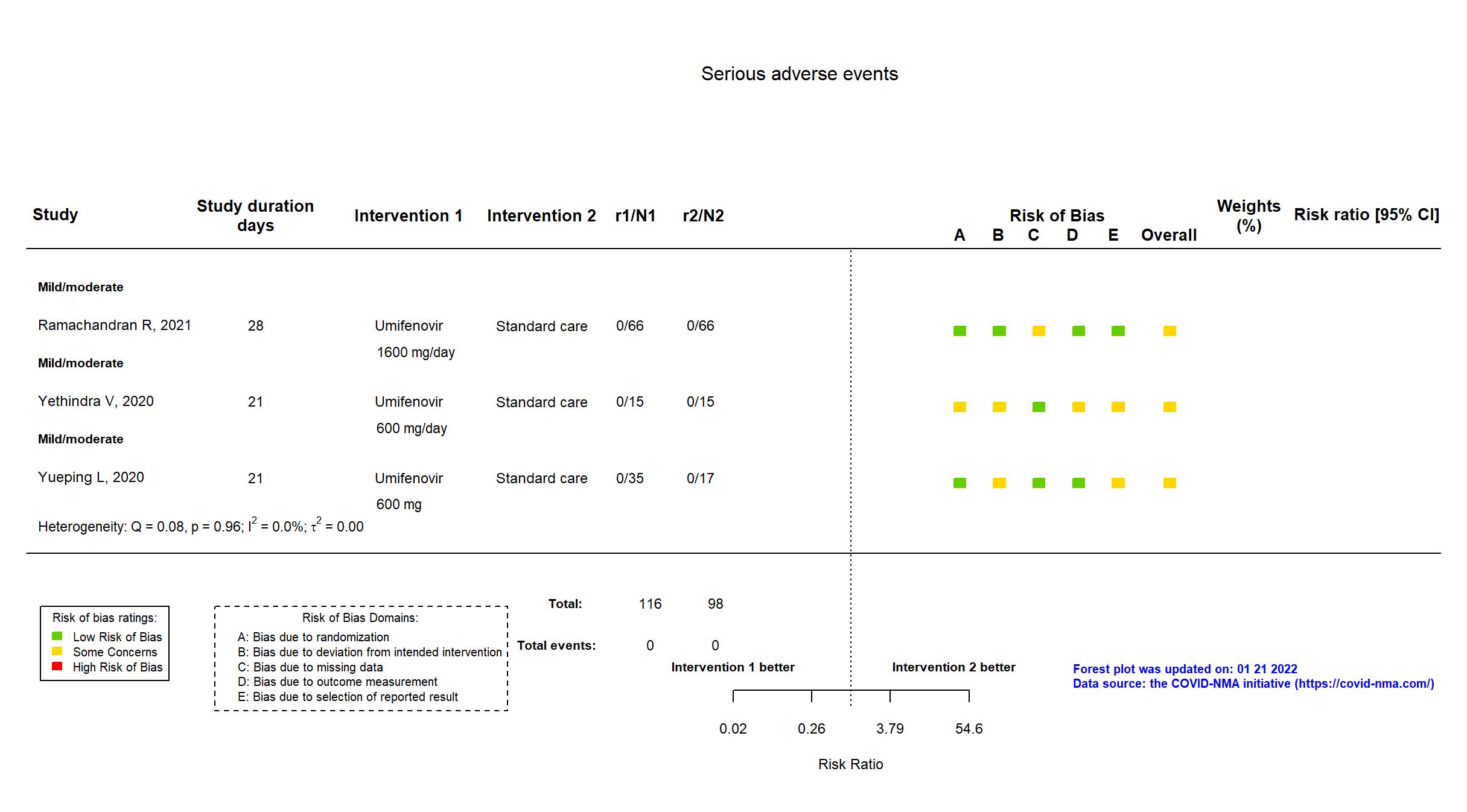
FOREST PLOTS -2022-01-21






Trial CTRI/2020/09/027535
Publication Ramachandran R, Int. J. Infect. Dis. (2021) (published paper)
Dates: 2020-10-03 to 2021-04-28
Funding: Public/non profit (Council of Scientific and Industrial Research, Ministry of Science and Technology, Government of India)
Conflict of interest: No
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / India Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Umifenovir 800 mg orally twice daily for 14 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Umifenovir=66 Standard care=66 | |
| Characteristics of participants N= 132 Mean age : NR 92 males Severity : Mild: n=* / Moderate: n=41 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register Time from randomization to nasopharyngeal swab negativity by RT-PCR tests; For moderate patients: time to improvement by one category from randomisation on the eight-category ordinal scale defined by WHO & average change in the ordinal scale from baseline | |
| In the report Mild-asymptomatic patients: Time from randomization to nasopharyngeal swab negativity by two RT-PCR tests, for SARS-Cov-2 antigens, taken 24 hours apart; Moderate patients: time to improvement by one category from randomisation on the eight-category ordinal scale defined by WHO & average change in the ordinal scale from baseline | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: N |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the study registry and protocol were used in data extraction and risk of bias assessment. The study achieved the target sample size specified in the trial registry. There is no change from the trial registration in the intervention and control treatments. The registry primary outcomes reflect the reported primary outcomes. Some outcomes (e.g. mortality) are reported in the paper, but was not pre-specified in the trial registry/protocol. Participants were stratified at randomization and analyzed by severity at baseline (asymptomatic + mild or moderate severity). |
Trial *
Publication Yethindra V, Int J Res Pharm Sci (2020) (published paper)
Dates: 2020-03-12 to 2020-05-11
Funding: No specific funding (Not applicable)
Conflict of interest: No
| Methods | |
| RCT | |
| Location :
Single center / Kyrgyzstan Follow-up duration (days): 21 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Umifenovir 200 mg orally 3 times a day for 1-5 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Umifenovir=15 Standard care=15 | |
| Characteristics of participants N= 30 Mean age : NR 18 males Severity : Mild: n=* / Moderate: n=* / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register NR | |
| In the report Time to clinical recovery (TTCR), a composite of body temperature and cough amelioration over 72 h. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | Only the published article was used in data extraction and assessment of risk of bias. No trial registry, protocol or statistical analysis plan was available. Limited data to assess RoB (no information on blinding of the participants/carers/outcome assessors), and insufficient data on baseline characteristics of 30 participants included in the study. |
Trial NCT04252885
Publication Yueping L, CellPress (2020) (published paper)
Dates: 01feb2020 to 28mar2020
Funding: Public/non profit (Project 2018ZX10302103-002, 2017ZX10202102-003-004 and Infectious Disease Specialty of Guangzhou High-level Clinical Key Specialty (2019-2021) )
Conflict of interest: No
| Methods | |
| RCT Blinding: Participants | |
| Location :
Single center / China Follow-up duration (days): 21 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Lopinavir-Ritonavir LPV/r: 200/50 mg orally twice a day for 7-14 days Umifenovir 200 mg orally 3 times a day for 7-14 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Lopinavir-Ritonavir=34 Umifenovir=35 Standard care=17 | |
| Characteristics of participants N= 86 Mean age : NR 40 males Severity : Mild: n=11 / Moderate: n=75 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register The rate of virus inhibition [ Time Frame: Day 0, 2, 4, 7, 10, 14 and 21 ] | |
| In the report Time of positive-to-negative conversion of SARS-CoV-2 nucleic acid from initiating treatment to day 21, with the enrollment day as the first day of treatment | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: No |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to all available versions of the published/pre-print article, the study registry and the reply provided by authors were used in data extraction and risk of bias assessment.The study did not achieve the target sample size specified in the trial registry. There is no change from the trial registration in the intervention and control treatments. The primary outcome indicated in the registry is measured at multiple time points, some of which are not reported in the paper. Some outcomes are reported in the paper, but were not pre-specified in the trial registry/protocol (e.g., adverse events). |