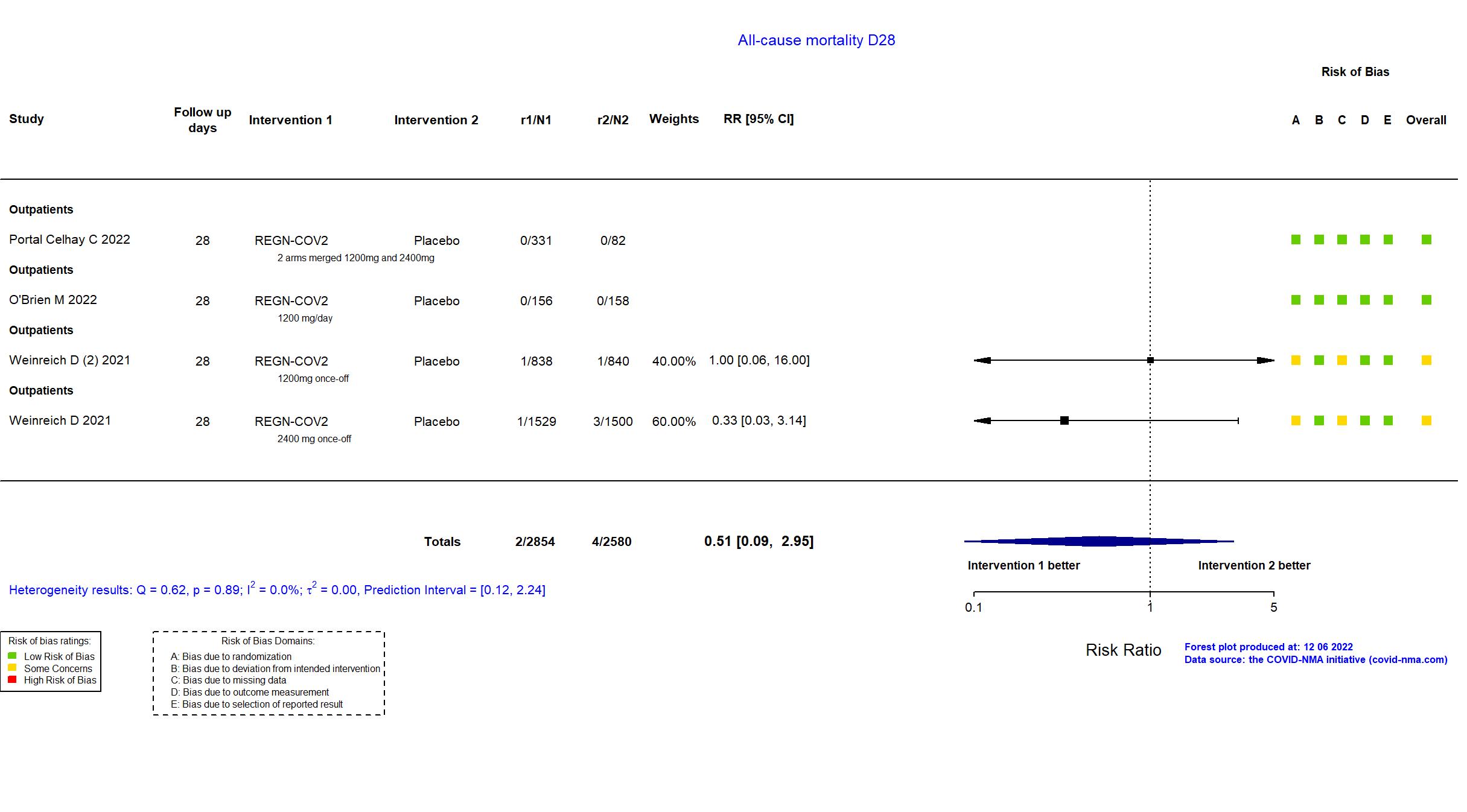
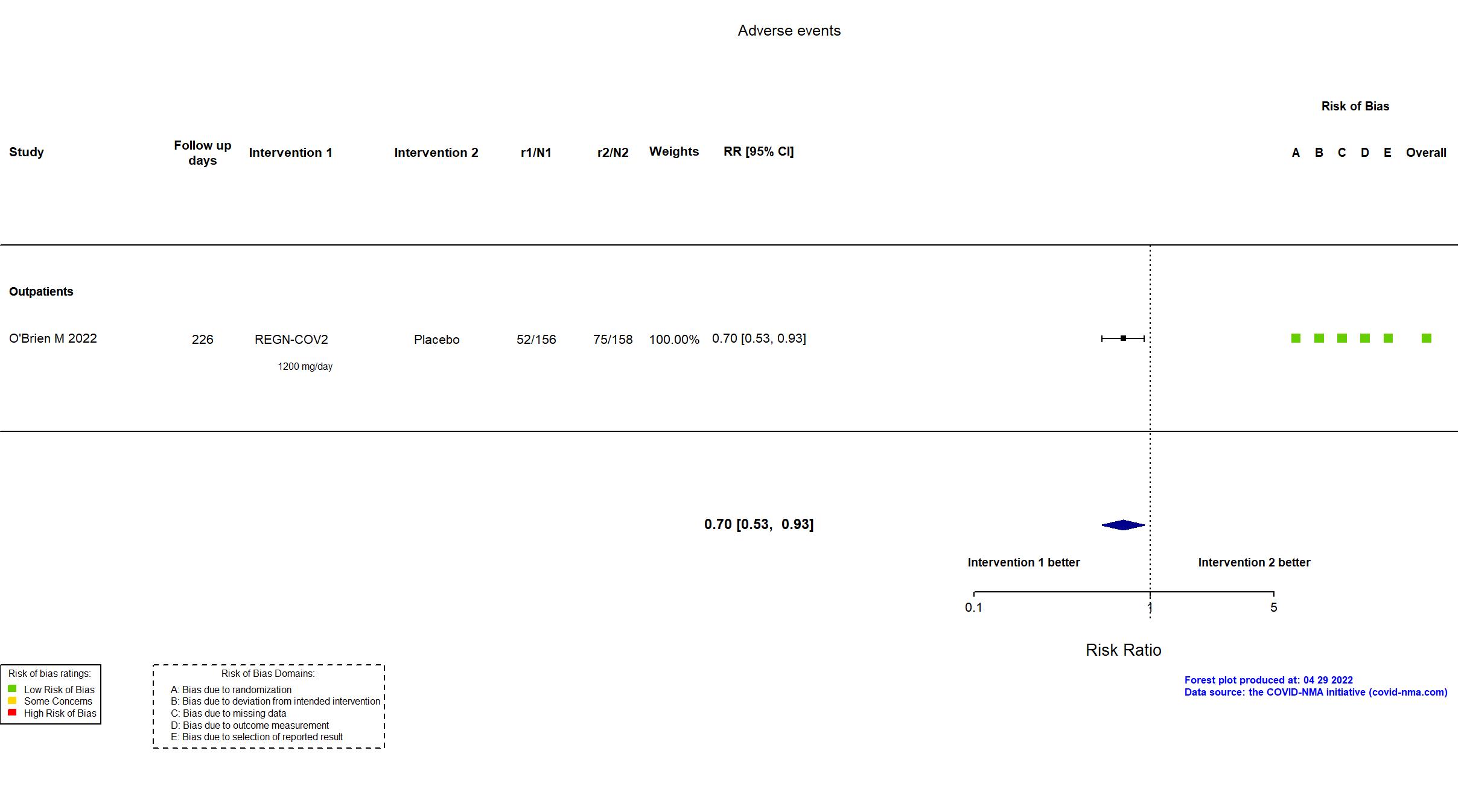
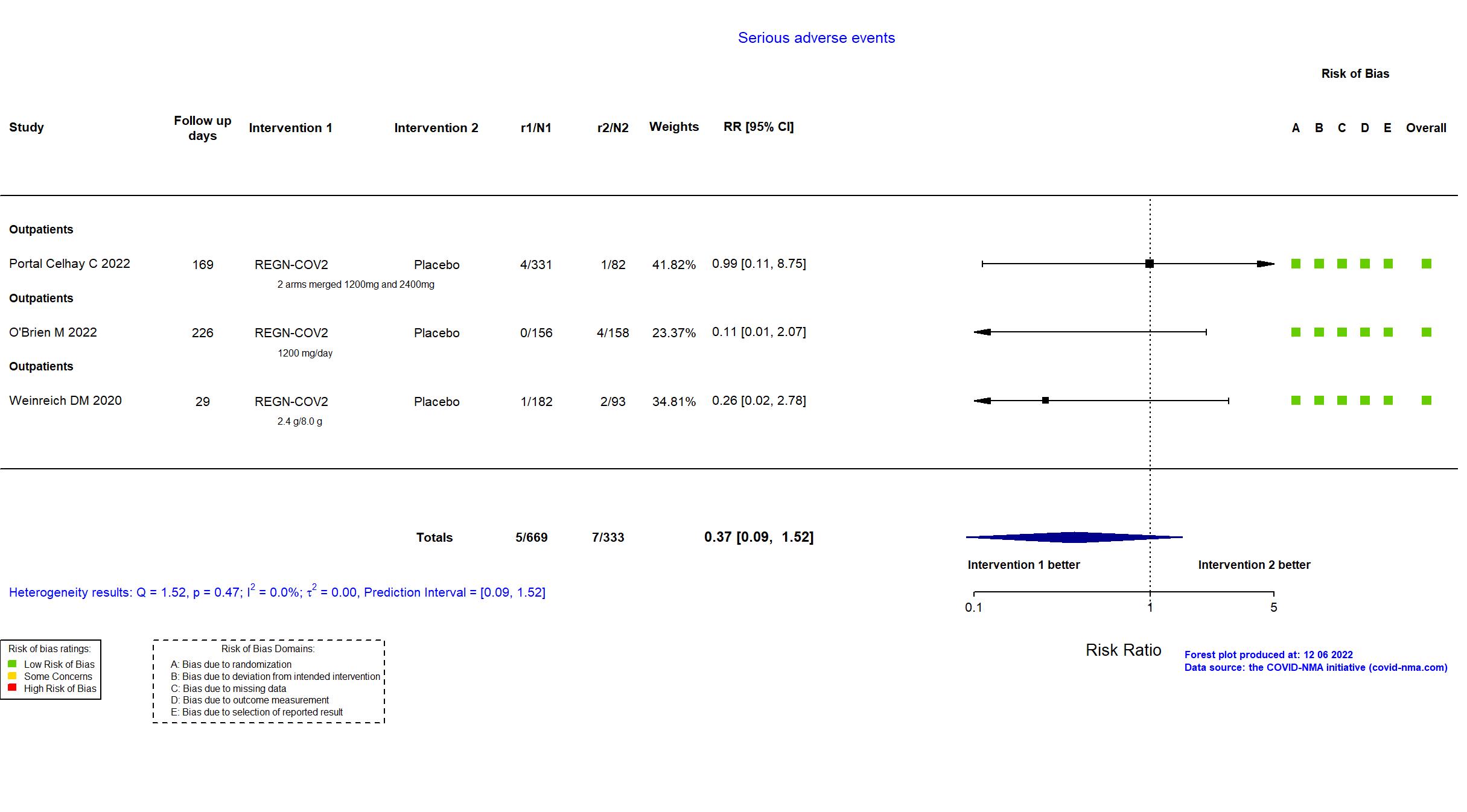
Casirivimab+Imdevimab (REGN-COV2) vs Standard care/Placebo (RCT)
Mild outpatients
FOREST PLOTS -2022-12-06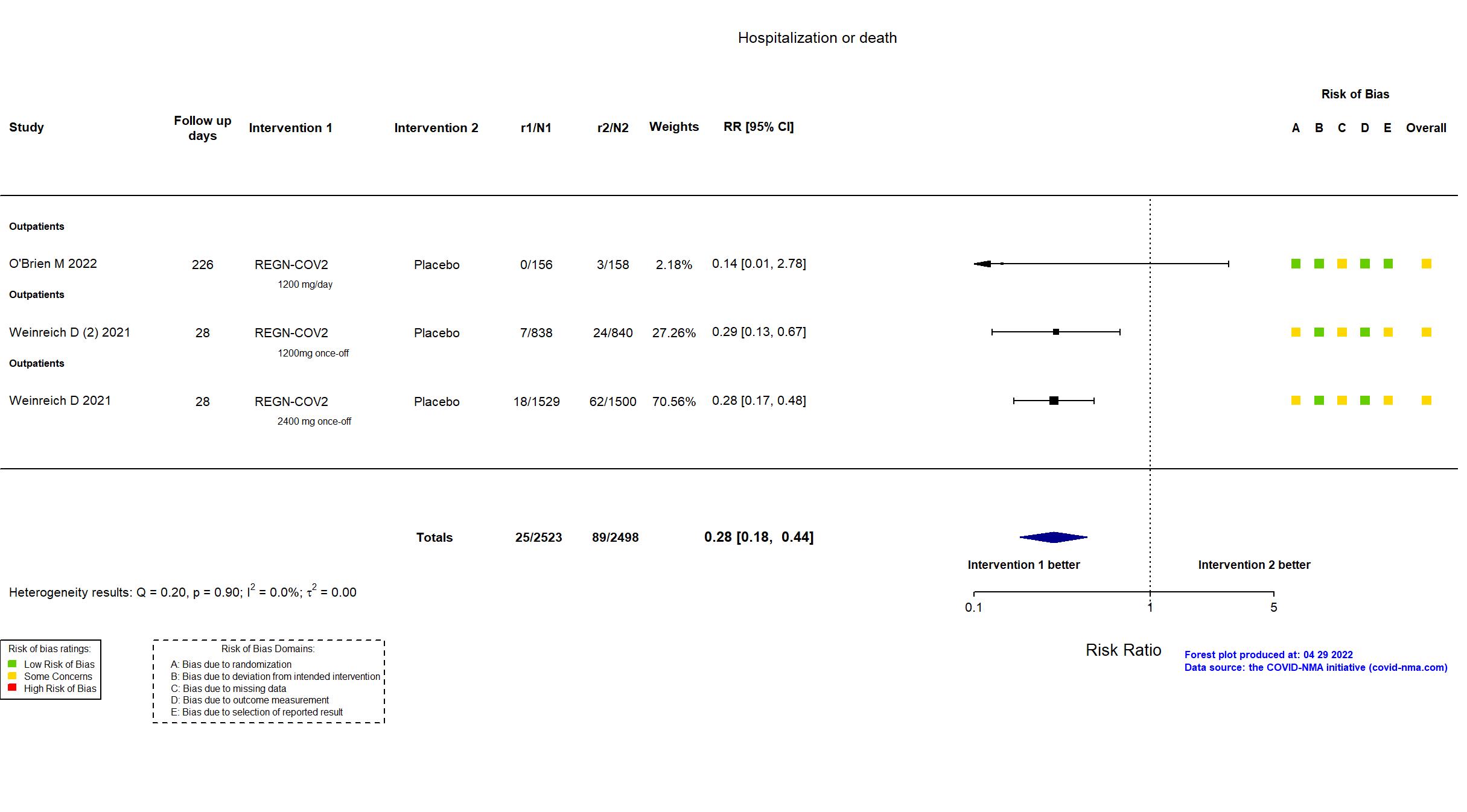







Trial NCT04452318
Publication O'Brien M, JAMA (2022) (published paper)
Dates: 2020-07-13 to 2021-01-28
Funding: Mixed (Regeneron Pharmaceuticals, Inc.; F. Hoffmann-La Roche Ltd.; COVID-19 Prevention Network (CoVPN) )
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / Moldova, Romania, USA Follow-up duration (days): 226 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
REGN-COV2 1200 mg subcutaneously once-off on day 1 |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : REGN-COV2=156 Placebo=158 | |
| Characteristics of participants N= 314 Mean age : NR 141 males Severity : Mild: n= 0/ Asymptomatic: n=314 | |
| Primary outcome | |
| In the register Proportion of participants who subsequently develop signs and symptoms (broad-term) within 14 days of a positive RT-qPCR at baseline or during the EAP [ Time Frame: Up to 1 month ] | |
| In the report The proportion of participants who had a positive RT-qPCR result at baseline or during the 28-day efficacy assessment period and who developed signs and symptoms of COVID-19 within 14 days of the positive RT-qPCR result. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published report, the 2 versions of the pre-print article, the prospective registry (2020-06-30), protocol and supplementary appendices (including supplementary methods and statistical analysis methods) were used in data extraction and assessment of risk of bias. The original phase 1-3 protocol was available. There were no substantive differences between the population, procedures, interventions or outcomes in the pre-print article and supplementary appendices and the final registry. Considerable changes were made to outcomes in the registry during and after the trial, but these do not affect the data extracted. The article reports the adult/adolescent treatment part of a larger two-part trial that includes both treatment of asymptomatic RT-PCR-positive COVID patients (adults/adolescents and children) to prevent development of symptoms and prophylaxis in RT-PCR-negative contacts (adults/adolescents and children) of confirmed cases. The article also reports the proportion of participants who had a hospitalization related to a confirmed SARS-CoV-2 infection up to day 29. This was used in the composite hospitalization or death outcome extracted.
Of note, "the trial was ongoing at the time of this report, so while all participants completed the 28-day efficacy assessment period, some were early in the follow-up period". Hence, all data were extracted under the day 28 timepoint until the updated completed report is available. This study was updated on October 6th, 2021 with data from the updated pre-print article and data gained from contact with authors. This study was updated on March 2nd, 2022 with data from the published report. |
Trial NCT04666441
Publication Portal Celhay C, JAMA Netw Open (2022) (results posted on registry)
Dates: 2020-12-15 to 2021-02-26
Funding: Private (Regeneron Pharmaceuticals, Inc, and Hoffman-La Roche. Employees of Regeneron Pharmaceuticals participated in the development and review of the manuscript.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: triple blinding | |
| Location :
Multicenter / USA Follow-up duration (days): 169 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
REGN-COV2 2400mg Casirivimab 1200 mg + Imdevimab 1200 mg intravenously single dose REGN-COV2 1200mg Casirivimab 600 mg + Imdevimab 600 mg intravenously single dose REGN-COV2 Casirivimab 600 mg + Imdevimab 600 mg intravenously single dose |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=82 REGN-COV2 2400mg=166 REGN-COV2 1200mg=165 REGN-COV2=331 | |
| Characteristics of participants N= 744 Mean age : NR 292 males Severity : Mild: n= */ Asymptomatic: n=* | |
| Primary outcome | |
| In the register Time-Weighted Average Daily Change From Day 1 in Viral Load in NP Swab Samples [ Time Frame: Day 1 to Day 7 ] Time-weighted average daily change from Day 1 in viral load (log10 copies/mL), as measured by reverse transcription quantitative polymerase chain reaction (RT-qPCR) in nasopharyngeal (NP) swab samples. | |
| In the report Time-weighted average daily change from baseline (TWACB) in viral load (log10 copies per milliliter) to day 7, as measured by RT-qPCR of NP swab samples in patients who had a central laboratory–determined RT-qPCR positive test at baseline and were seronegative (ie, negative for anti-spike [S1] IgA, anti-spike [S1] IgG, and anti-nucleocapsid IgG) | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: N |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment | In addition to the published article, the protocol, statistical analysis plan, supplemental materials and study registry were used in data extraction and risk of bias assessment. Only the interventions and placebo arms given IV were extracted. Registry posted results were extracted as the report published only interim results (at day 7, early database lock in Feb 2021, whereas the registry has longer follow-up results, posted Apr 2022, and reports on a larger sample size). The registry primary outcome reflects the reported primary outcome. Some outcomes (e.g. total adverse events, serious adverse events, mortality) are reported in the paper, and were pre-specified in the protocol but not the registry. Adverse events were reported in the publication reporting interim results but were not extractable from the registry with longer follow up. Viral negative conversion event were reported as well but data was presented for the pooled IV/SC placebo and could not be extracted. |
Trial NCT04425629
Publication Weinreich D, N Engl J Med (2021) (published paper)
Dates: 2020-09-24 to 2021-01-17
Funding: Mixed (Regeneron Pharmaceuticals, Inc.; Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / Mexico, USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
REGN-COV2 2400 mg IV once-off on day 1 |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : REGN-COV2=1529 Placebo=1500 | |
| Characteristics of participants N= 3029 Mean age : NR 1289 males Severity : Mild: n= 2696/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Proportion of patients with at least one (≥1) COVID-19-related hospitalization or all-cause death [ Time Frame: Through Day 29 ] | |
| In the report The proportion of patients with ≥1 Covid-19-related hospitalization or all-cause death through day 29 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the pre-print article, the prospective study registry, the supplementary appendices, protocol and statistical analysis plan were used in data extraction and risk of bias assessment. The overall study was a phase 1/2/3 trial recruiting 3 cohorts of COVID-19 outpatients: patients ≥18 years, patients <18 years, and patients pregnant at randomization. Initially, patients were randomized to receive either IV placebo, REGEN-COV 2400mg (1200mg each of casirivimab and imdevimab), or REGEN-COV 8000mg (4000mg each antibody). Based upon phase 1/2 results, the trial was amended so that subsequent patients enrolled all had ≥1 risk factor for severe Covid-19 and were randomized to receive either IV placebo, REGEN-COV 1200mg (600mg each antibody) IV, or REGEN-COV 2400mg. In February 2021, an independent data monitoring committee recommended stopping enrolment of patients into the placebo group because the accumulated data indicated efficacy of REGEN-COV. The reported analysis included only patients ≥ 18 years of age with ≥1 risk factor for severe COVID-19, randomized to REGEN-COV 2400mg or 1200mg, with their concurrent placebo groups serving as a control. Patients randomized in the early stages of the trial who had no risk factors for severe COVID-19 were excluded from analyses. There is no change from the trial registration in the intervention and control treatments. The primary outcomes indicated in the registry reflects the primary outcome reported in the paper. Some outcomes from the registry are not reported in the paper (e.g., proportion of participants requiring supplemental oxygen due to COVID-19 by day 29, all-cause death by day 120). |
Trial NCT04425629
Publication Weinreich D (2), N Engl J Med (2021) (published paper)
Dates: 2020-09-24 to 2021-01-17
Funding: Mixed (Regeneron Pharmaceuticals, Inc.; Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / Mexico, USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
REGN-COV2 1200 mg IV once-off on day 1 |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : REGN-COV2=838 Placebo=840 | |
| Characteristics of participants N= 1678 Mean age : NR 716 males Severity : Mild: n= 1484/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Proportion of patients with at least one (≥1) COVID-19-related hospitalization or all-cause death [ Time Frame: Through Day 29 ] | |
| In the report The proportion of patients with ≥1 Covid-19-related hospitalization or all-cause death through day 29. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the pre-print article, the prospective study registry, the supplementary appendices, protocol and statistical analysis plan were used in data extraction and risk of bias assessment. . The overall study was a phase 1/2/3 trial recruiting 3 cohorts of COVID-19 outpatients: patients ≥18 years, patients <18 years, and patients pregnant at randomization. Initially, patients were randomized to receive either IV placebo, REGEN-COV 2400mg (1200mg each of casirivimab and imdevimab), or REGEN-COV 8000mg (4000mg each antibody). Based upon phase 1/2 results, the trial was amended so that subsequent patients enrolled all had ≥1 risk factor for severe Covid-19 and were randomized to receive either IV placebo, REGEN-COV 1200mg (600mg each antibody) IV, or REGEN-COV 2400mg. In February 2021, an independent data monitoring committee recommended stopping enrollment of patients into the placebo group because the accumulated data indicated efficacy of REGEN-COV. The reported analysis included only patients ≥ 18 years of age with ≥1 risk factor for severe COVID-19, randomized to REGEN-COV 2400mg or 1200mg, with their concurrent placebo groups serving as a control. Patients randomized in the early stages of the trial who had no risk factors for severe COVID-19 were excluded from analyses. There is no change from the trial registration in the intervention and control treatments. The primary outcomes indicated in the registry reflects the primary outcome reported in the paper. Some outcomes from the registry are not reported in the paper (e.g., proportion of participants requiring supplemental oxygen due to COVID-19 by day 29, all-cause death by day 120). |
Trial NCT04425629
Publication Weinreich DM, N Engl J Med (2020) (published paper)
Dates: 2020-06-16 to 2020-08-13
Funding: Mixed (Regeneron Pharmaceuticals; Biomedical and Advanced Research and Development Authority of the Department of Health and Human Services)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: | |
| Location :
Multicenter / USA Follow-up duration (days): 29 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
REGN-COV2 2.4 g/8.0 g in a 250 mL normal saline solution IV once off, over a period of 1 hour. |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : REGN-COV2=182 Placebo=93 | |
| Characteristics of participants N= 275 Mean age : NR 134 males Severity : Mild: n= 275/ Asymptomatic: n= | |
| Primary outcome | |
| In the register Proportion of patients with treatment-emergent serious adverse events (SAEs) [ Time Frame: Through Day 29 ] Proportion of patients with infusion-related reactions [ Time Frame: Through Day 4 ] Proportion of patients with hypersensitivity reactions | |
| In the report Time-weighted average change in viral load from baseline (day 1) through day 7 and the percentage of patients with at least one Covid-19–related medically attended visit through day 29 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment | In addition to the published article, the trial registry, protocol, statistical analysis plan and supplementary materials were used in data extraction. This is an interim analysis reporting early Phase I/II results from an ongoing operationally seamless (continual enrollment) Phase I/II/III trial. The data cutoff for this interim analysis was September 4, 2020. There were no substantive differences between the published article and the trial registry, protocol and statistical analysis plan in population, procedures and interventions. Several Phase I/II clinical outcomes included in the protocol were not reported. A fourth Phase II arm, REGN10989 included in the protocol dependent upon regulatory clearance, was not reported. The population in the interim report did not achieve the pre-stated Phase I/II sample size. Quote: "One center was found to have violations of Good Clinical Practice guidelines (not related to the collection of data on efficacy or safety end points) and was withdrawn from the trial after analyses had been completed." The study is updated under a new publication Weinreich D, medrxiv, 2021. We present only data on outcome serious adverse events since it was reported in Weinreich D, medrxiv, 2021. |