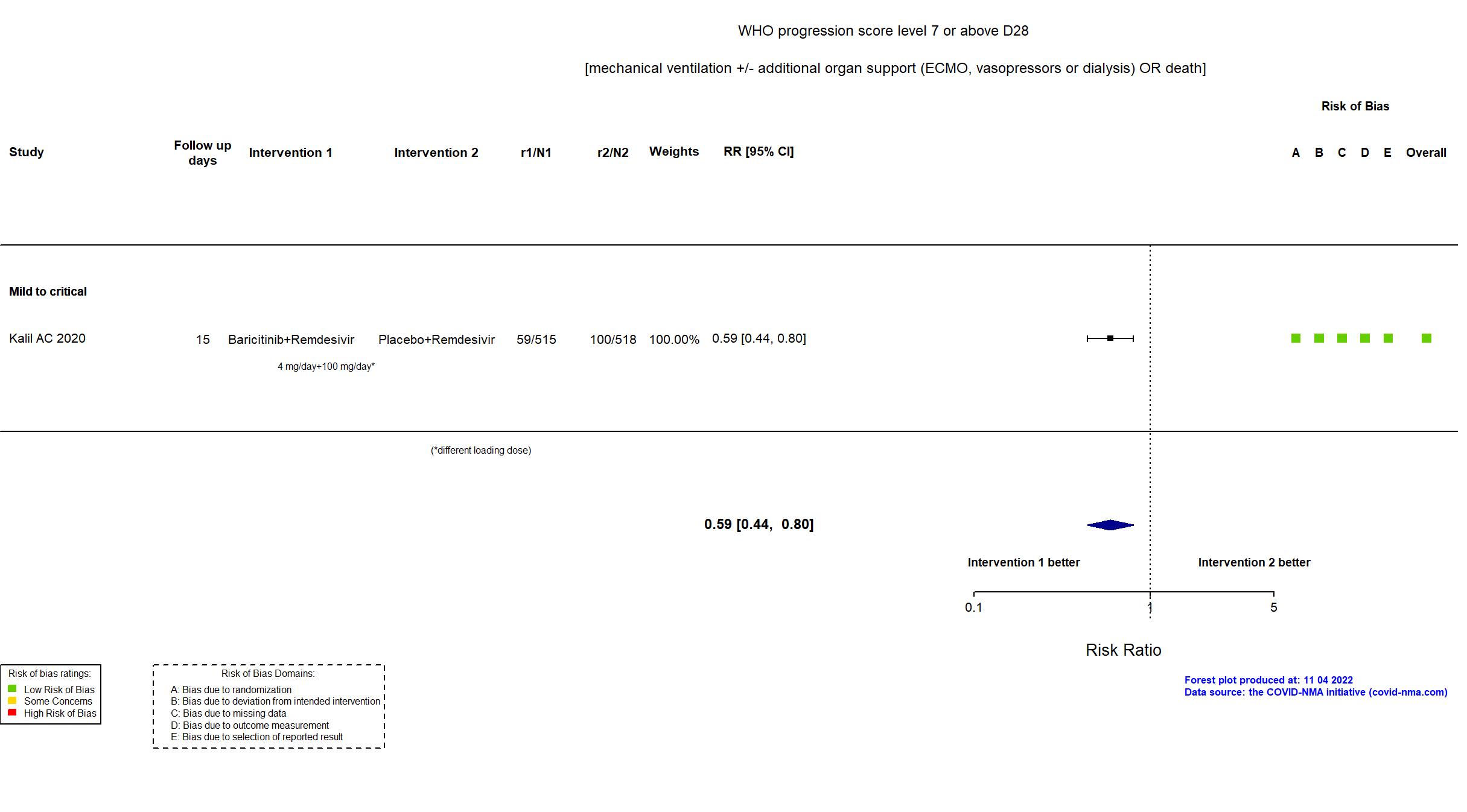
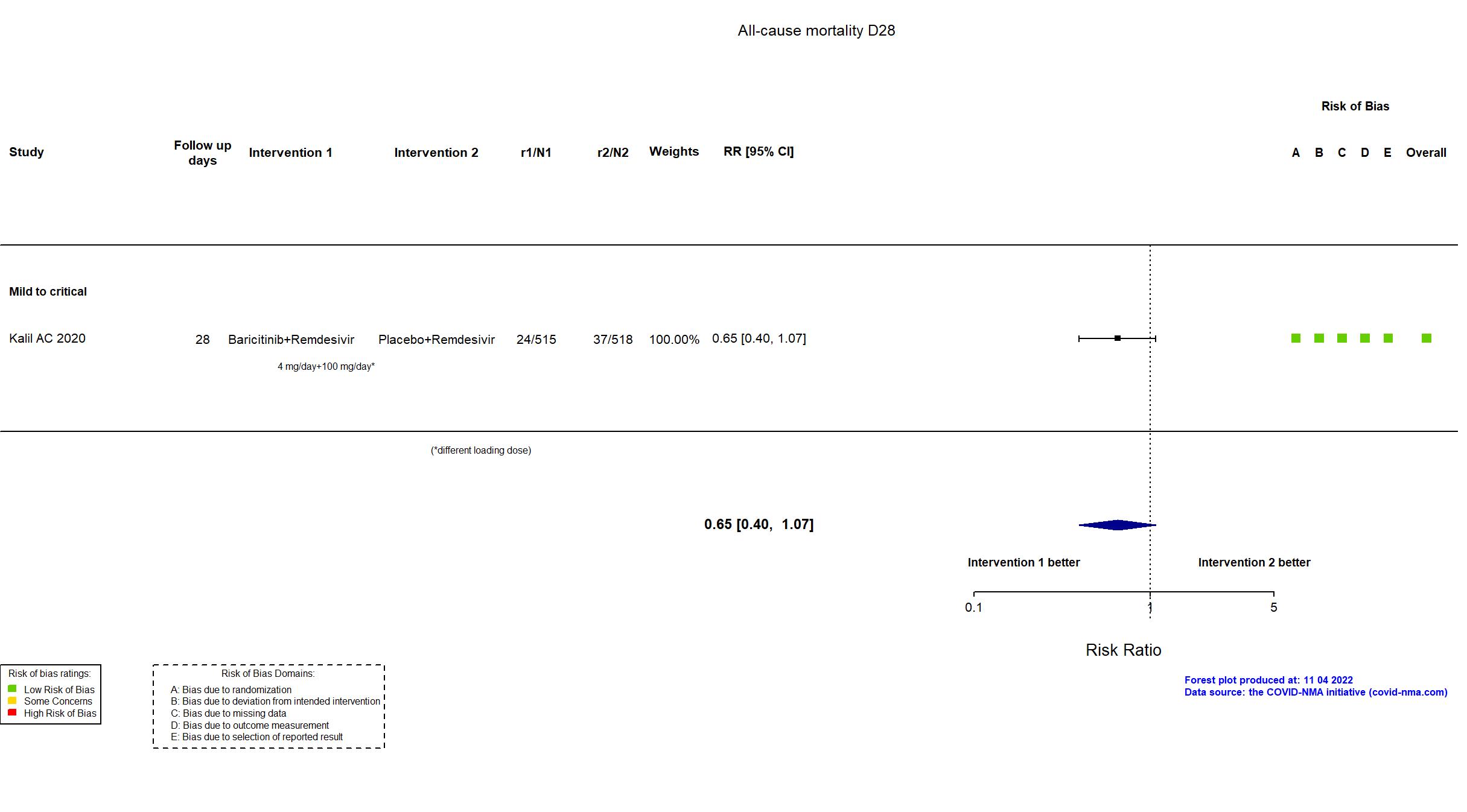
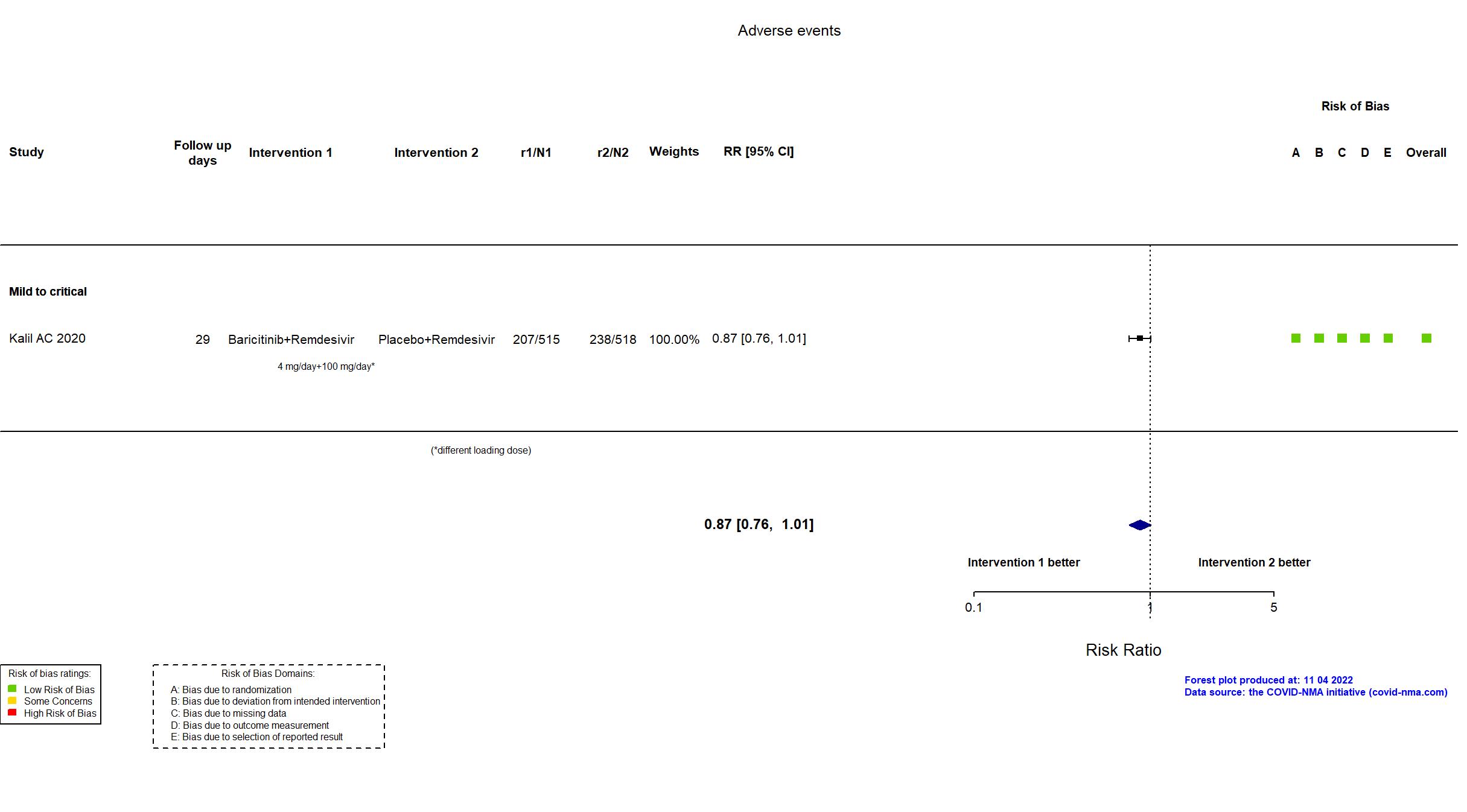
Baricitinib+Remdesivir vs Remdesivir (RCT)
Hospitalized patients
FOREST PLOTS -2022-11-04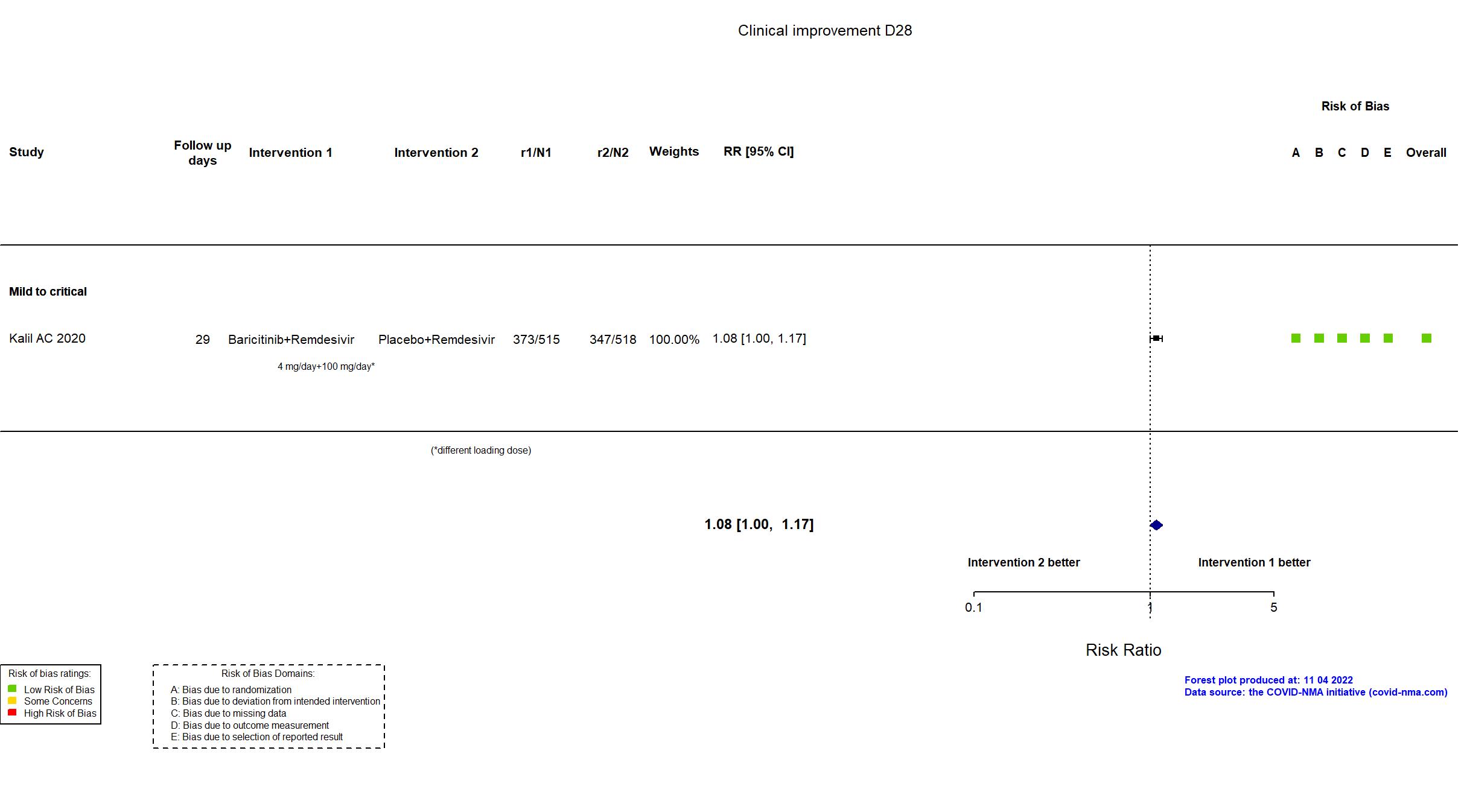
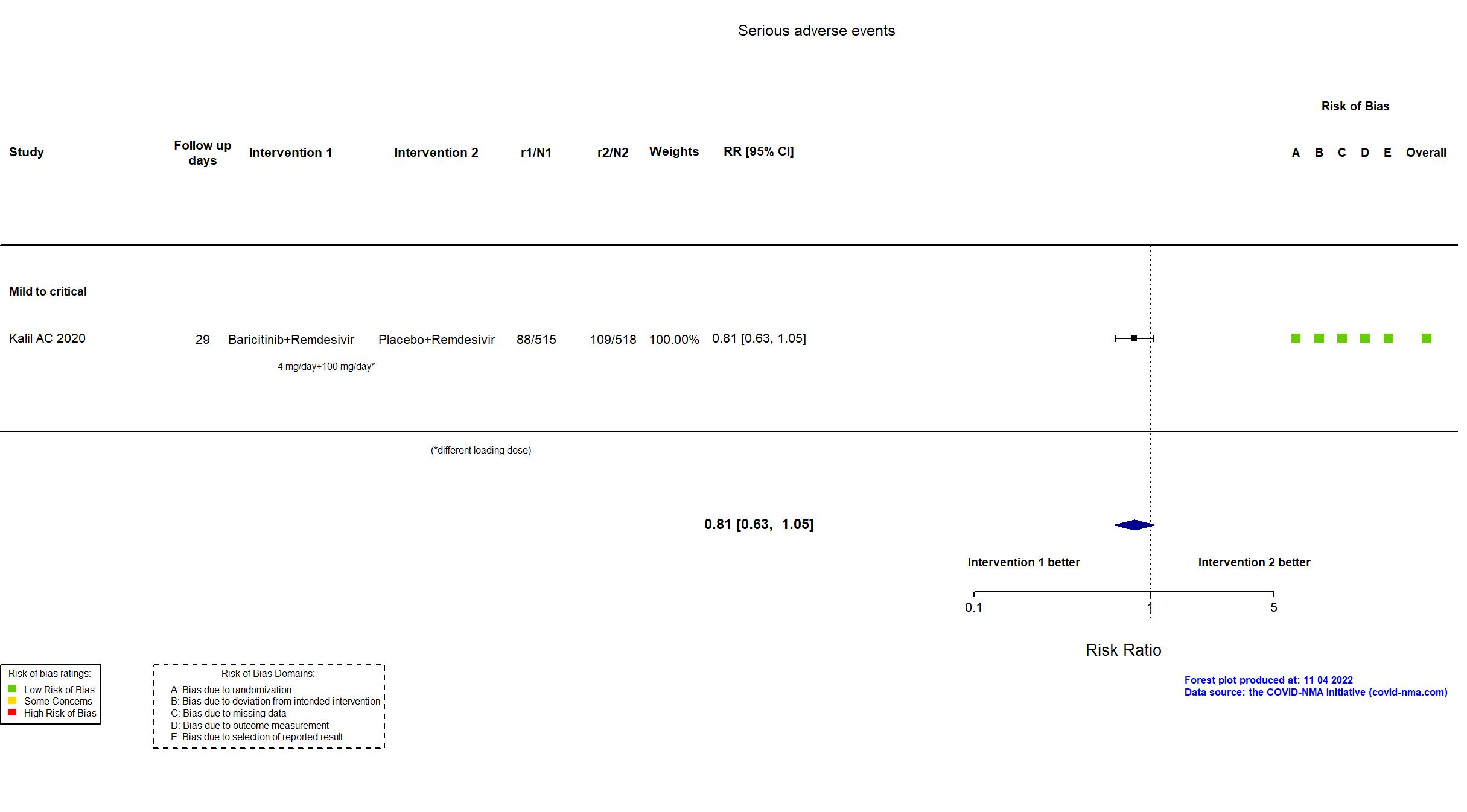
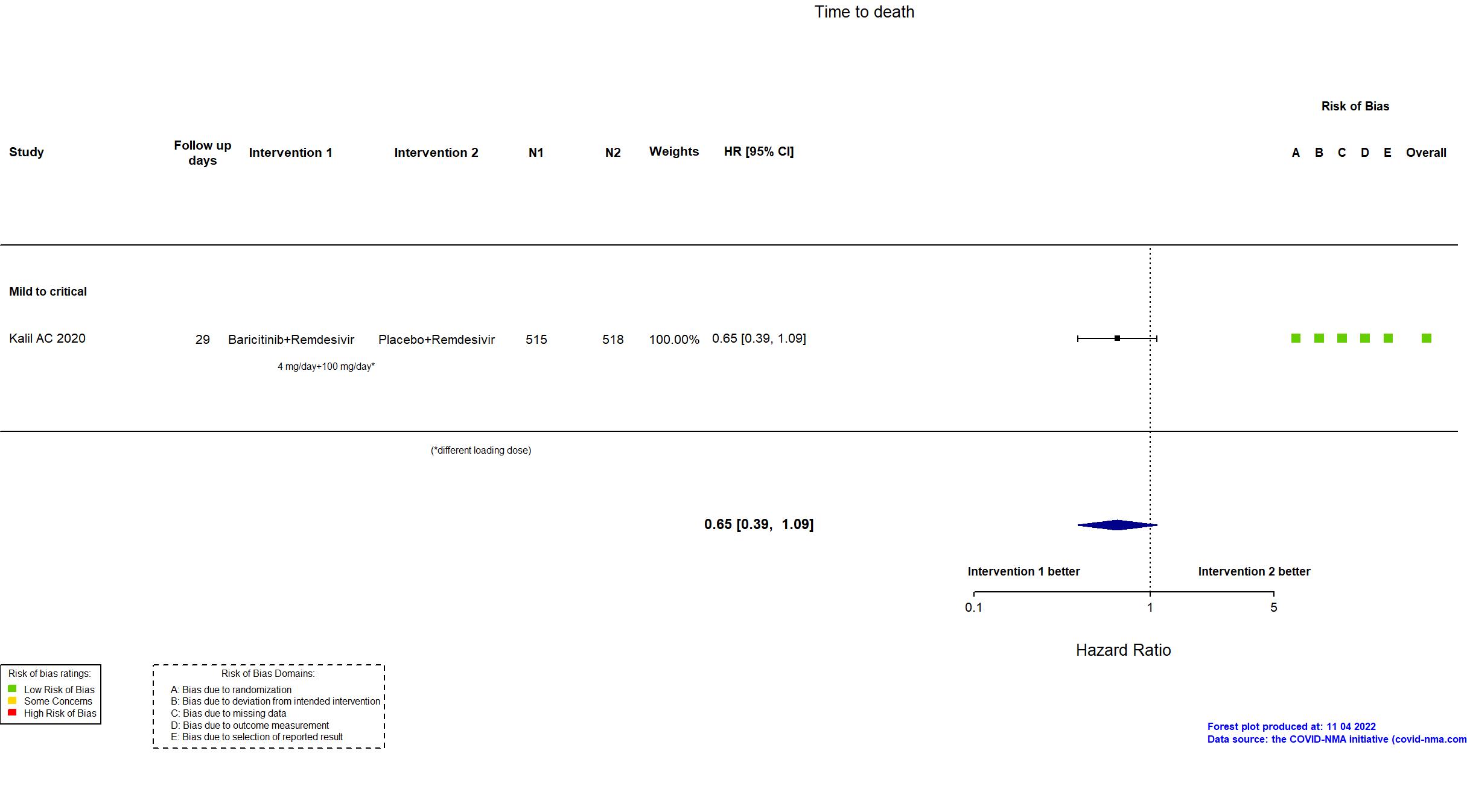






Trial NCT04401579
Publication ACTT-2 - Kalil AC, N Engl J Med (2020) (published paper)
Dates: 2020-05-08 to 2020-07-01
Funding: Public/non profit (National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), National Cancer Institute, NIH, Department of Defense, Defense Health Program, the governments of Japan, Mexico, Denmark, and Si)
Conflict of interest: No
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / Denmark, Japan, Mexico, Singapore, South Korea, Spain, UK, USA Follow-up duration (days): 29 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Baricitinib+Remdesivir Baricitinib: 4 mg orally or by nasogastric tube once a day for 14 days. Remdesivir: 200 mg IV on day 1 followed by 100 mg once a day for the next 9 days |
|
| Control
Placebo+Remdesivir 200 mg IV on day 1 followed by 100 mg once a day for the next 9 days | |
| Participants | |
| Randomized participants : Baricitinib+Remdesivir=515 Placebo+Remdesivir=518 | |
| Characteristics of participants N= 1033 Mean age : NR 652 males Severity : Mild: n=142 / Moderate: n=564 / Severe: n=216 Critical: n=111 | |
| Primary outcome | |
| In the register Time to recovery [ Time Frame: Day 1 through Day 29 ]; Time to Recovery by Race [ Time Frame: Day 1 through Day 29 ];Time to Recovery by Ethnicity [ Time Frame: Day 1 through Day 29 ];Time to Recovery by Sex [ Time Frame: Day 1 through Day 29 ] | |
| In the report Time to recovery, with the day of recovery defined as the first day, during the 28 days after enrollment, on which a patient attained category 1, 2, or 3 on the eight-category ordinal scale | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment |
In addition to the published article, the supplementary appendix, trial registry, study protocol and statistical analysis plan were used in data extraction and assessment of risk of bias. There were no substantive differences between the published article and the trial registry, study protocol and statistical analysis plan in study procedures, population and interventions. While most outcomes were reported as planned, RT-PCR results during the study follow up period were not. The study achieved its pre-stated sample size.
Data from a secondary analysis of this trial was published on April 27th 2022 (Fintzi J, Open Forum Infect Dis, 2022). On November 3rd, 2022, this study was updated with results published in the registry. |