Vitamin D vs Standard care/Placebo (RCT)
Hospitalized patients
FOREST PLOTS -2021-10-01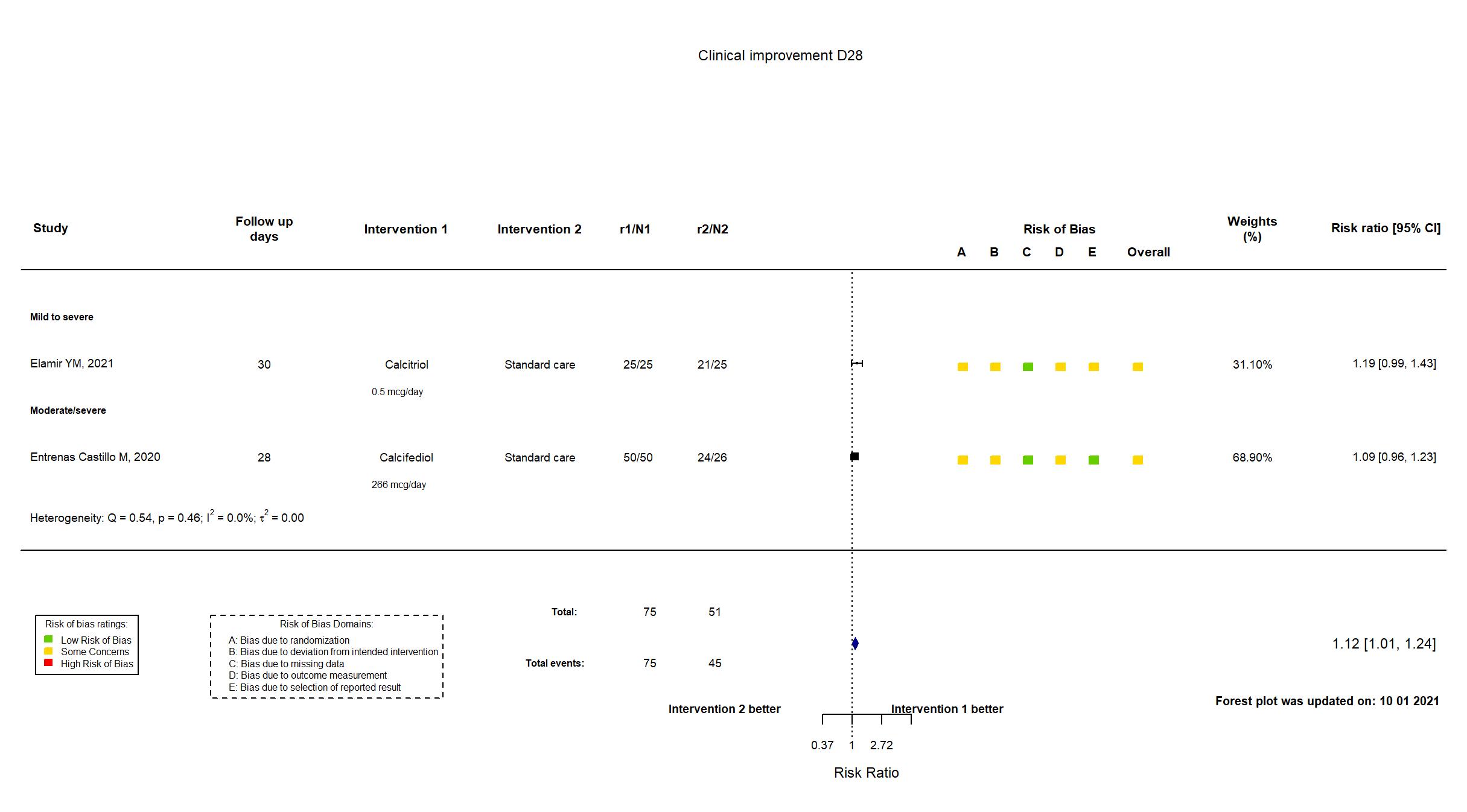
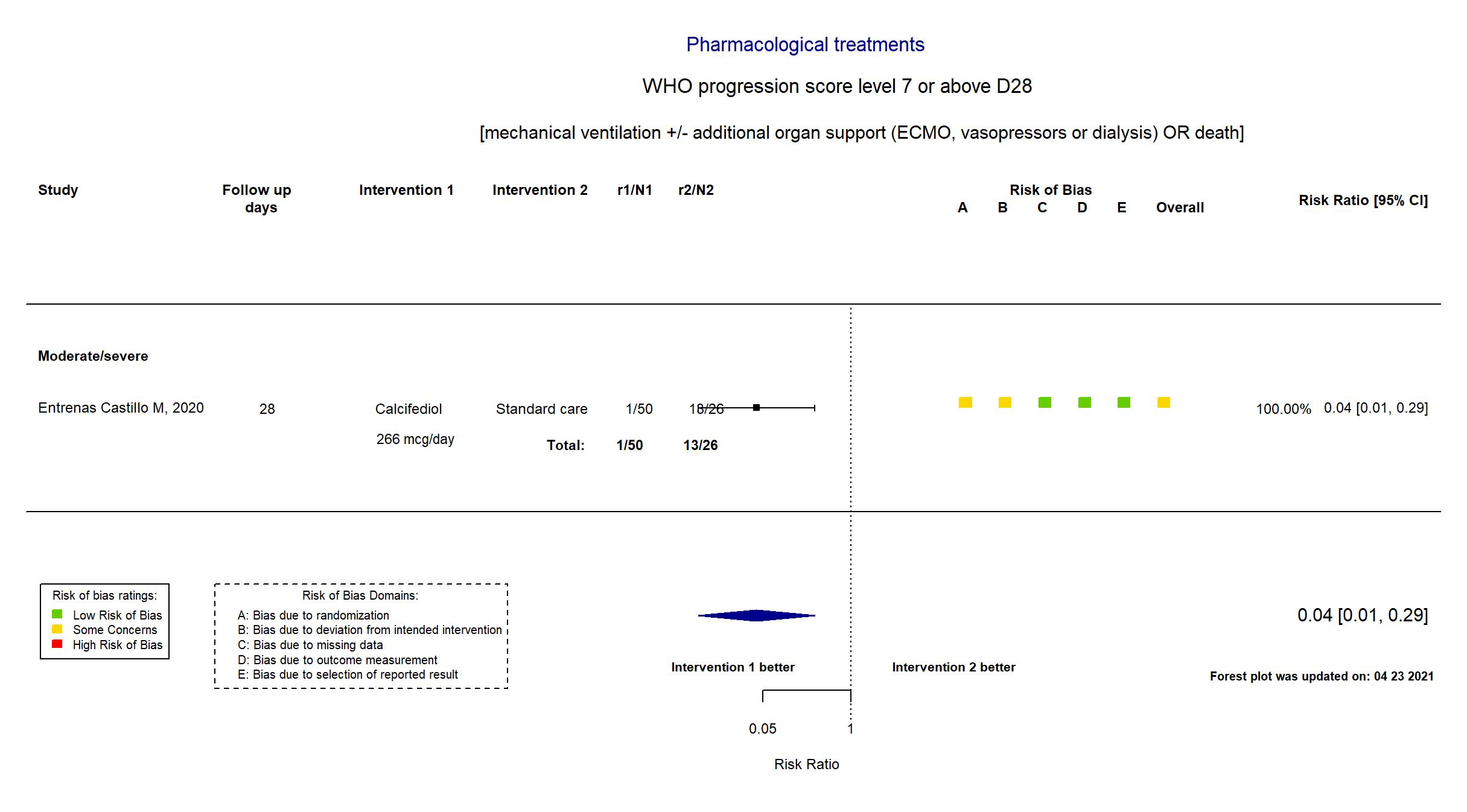
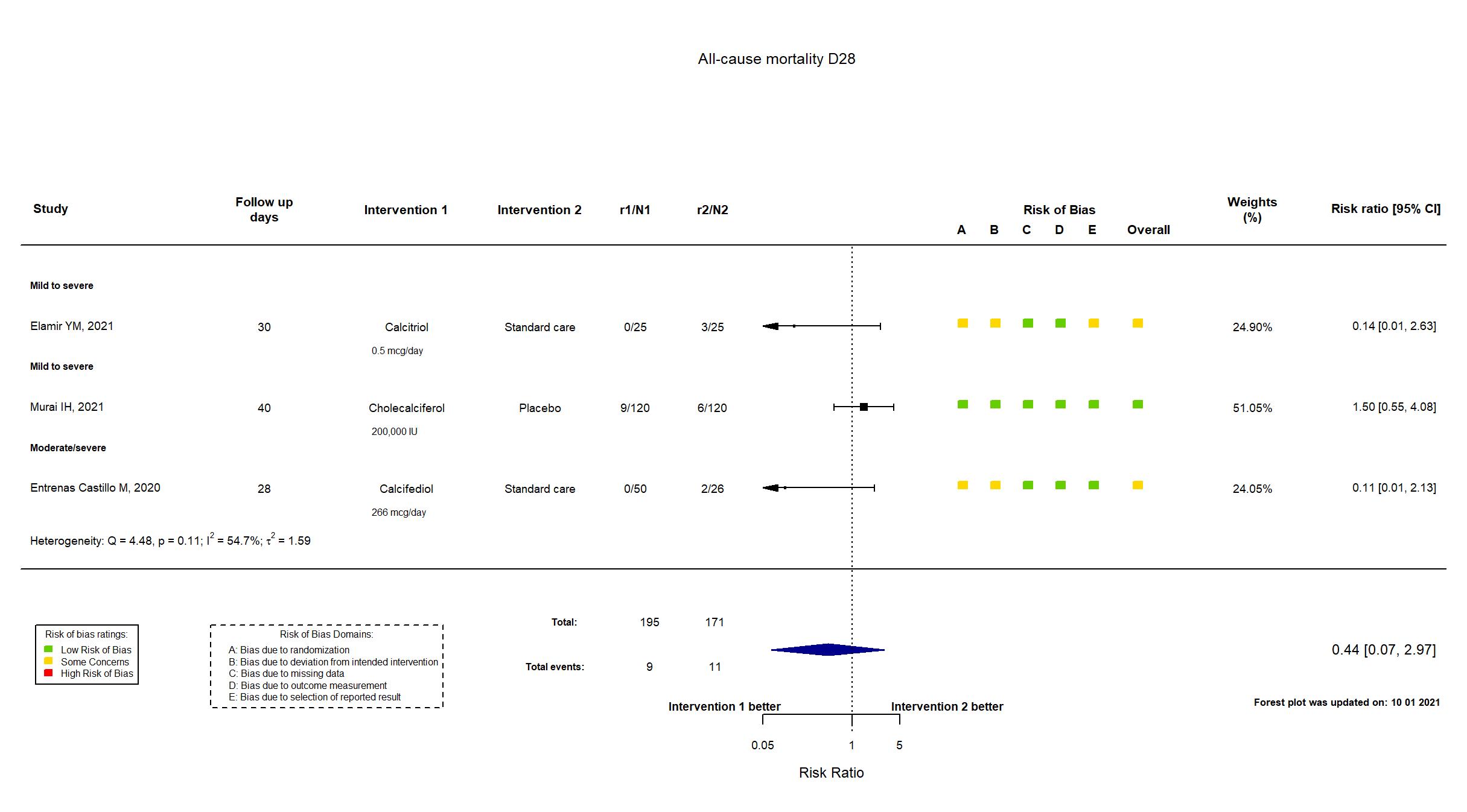
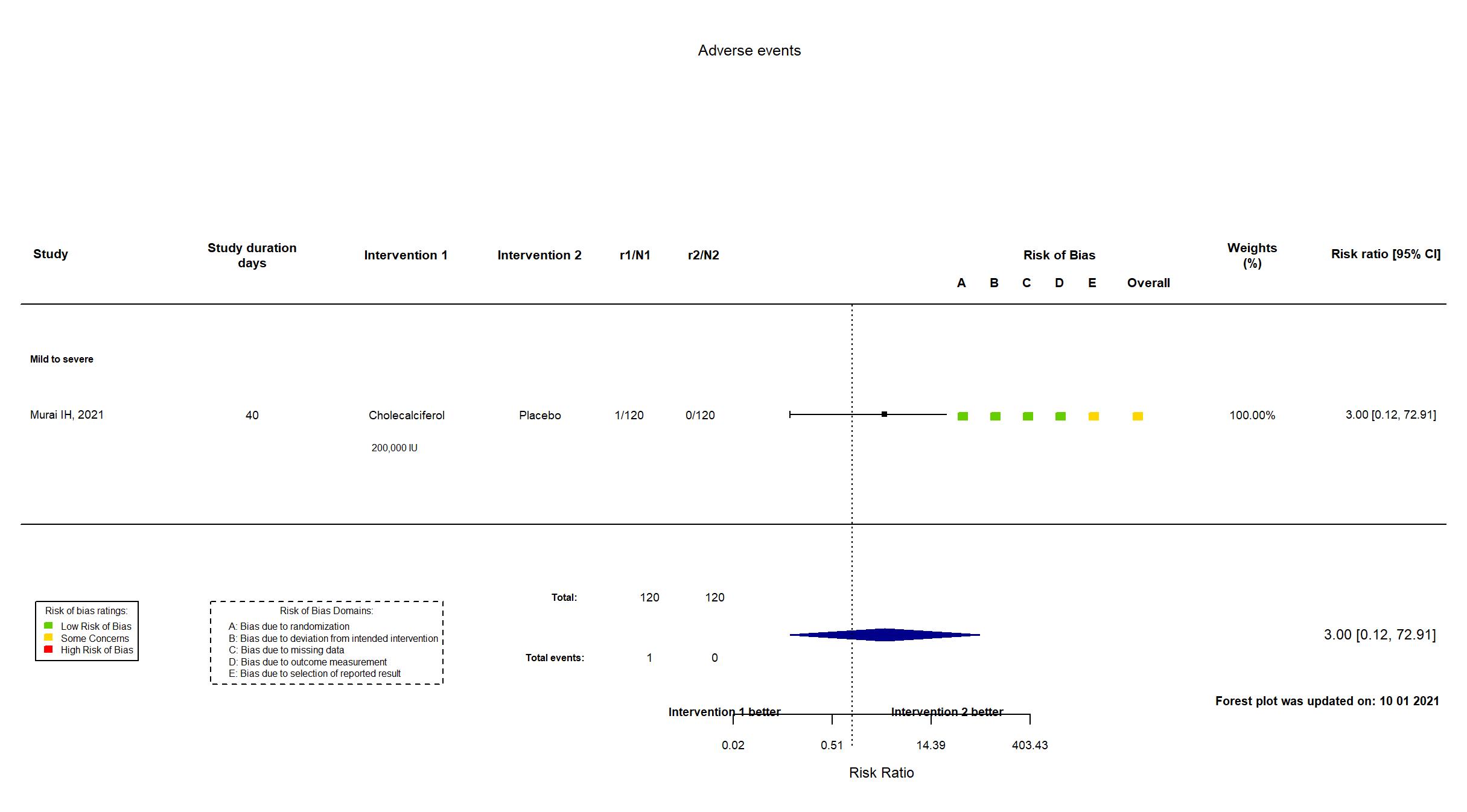





Trial *
Publication Elamir YM, Bone (2021) (published paper)
Dates: 2020-09-01 to 2020-12-30
Funding: Not reported/unclear
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / USA Follow-up duration (days): 30 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Calcitriol 0.5 mcg once a day for 14 days or until discharge, whichever came first |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Calcitriol=25 Standard care=25 | |
| Characteristics of participants N= 50 Mean age : NR 25 males Severity : Mild: n=24 / Moderate: n=20 / Severe: n=6 Critical: n=0 | |
| Primary outcome | |
| In the register NR | |
| In the report Outcomes of the effectiveness of calcitriol in the treatment of COVID19 included oxygen requirements, length of hospital stay, need for ICU admission, mortality, and readmission. | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | Only the published article was used in data extraction and assessment of risk of bias. No registry, protocol or statistical analysis plan was available. |
Trial NCT04366908
Publication Entrenas Castillo M, J Steroid Biochem Mo (2020) (published paper)
Funding: Public/non profit (Maimónides Biomedical Research Institute of Córdoba)
Conflict of interest: *
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / Spain Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Calcifediol 532 mcg orally on day 1, followed by 266 mcg on days 3 and 7 then weekly until discharge or ICU admission |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Calcifediol=50 Standard care=26 | |
| Characteristics of participants N= 76 Mean age : NR 45 males Severity : Mild: n=0 / Moderate: n=* / Severe: n=* Critical: n=0 | |
| Primary outcome | |
| In the register Proportion of subjects who enter the ICU; Proportion of subjects who die (Time Frame: At day 28) | |
| In the report Rate of ICU admission and death | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published report of the pilot study (journal pre-proof), the study registry was used in data extraction. There is no change from the trial registration in the intervention and control treatments. Pre-specified secondary outcomes included in the registry were not reported. Serum 25OHD concentrations at baseline or during treatment are not available. There were 2 deaths (control arm) out of 80 participants. It was not possible to find the exact timepoint for discharge, though not extracted. |
Trial NCT04449718
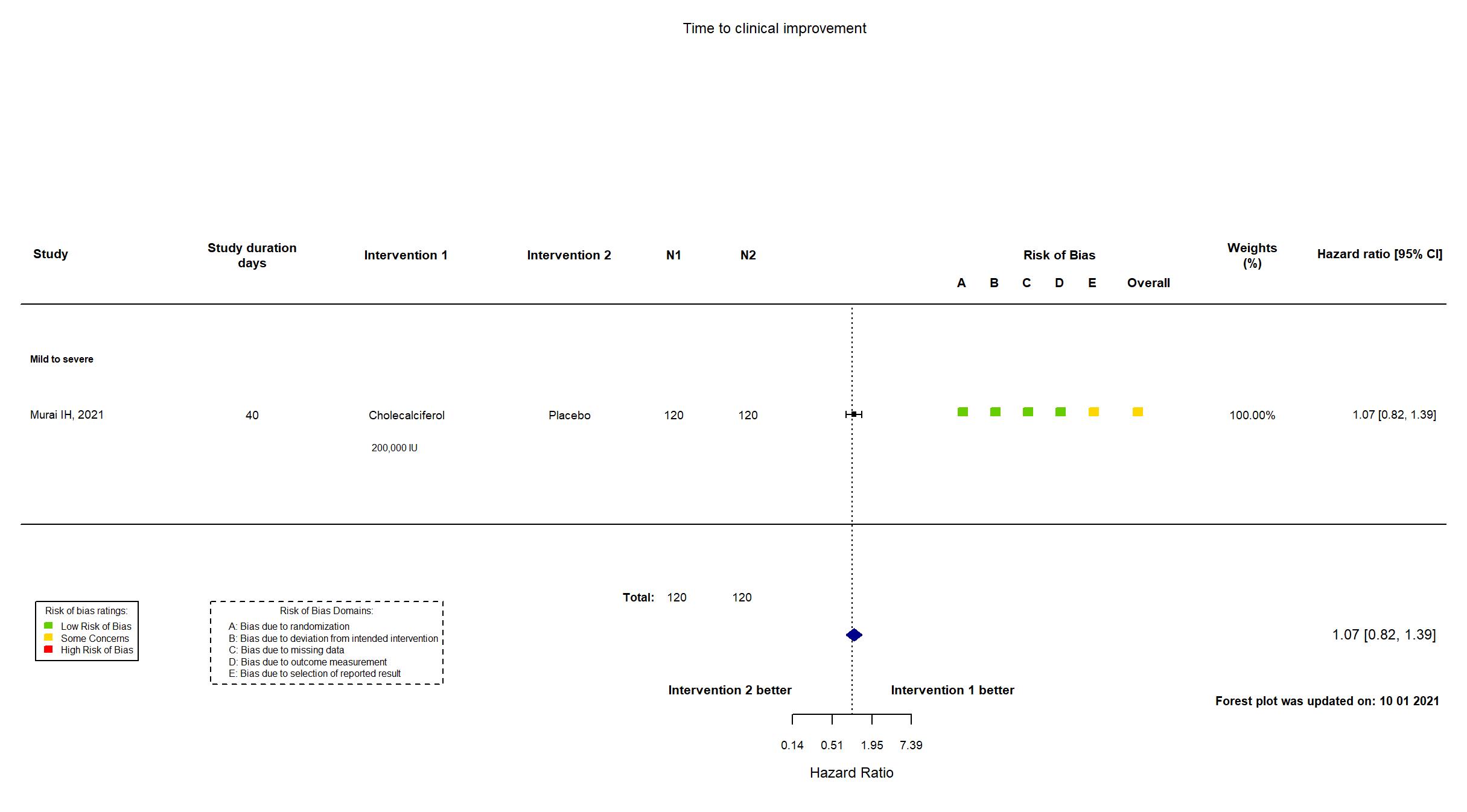
Publication Murai IH, Jama (2021) (published paper)
Dates: 2020-06-02 to 2020-08-27
Funding: Public/non profit (Sao Paulo Research Foundation (FAPESP); Conselho Nacional de Desenvolvimento Cientifico e Tecnologico)
Conflict of interest: No
| Methods | |
| RCT Blinding: Double blinded, no restrictions | |
| Location :
Multicenter / Brazil Follow-up duration (days): 40 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Cholecalciferol 200 000 IU orally dissolved in 10 mL of peanut oil solution once off |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Cholecalciferol =120 Placebo=120 | |
| Characteristics of participants N= 240 Mean age : NR 133 males Severity : Mild: n=25 / Moderate: n=181 / Severe: n=31 Critical: n=0 | |
| Primary outcome | |
| In the register Length of hospitalization [ Time Frame: From date of randomization until the date of hospital discharge or death, which is usually less than 1 month ] | |
| In the report Hospital length of stay, defined as the total number of days that patients remained hospitalized from the date of randomization until the date of hospital discharge. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published/pre-print article, the trial registry was used in data extraction and assessment of risk of bias. Although the article states that the protocol and statistical analysis plan will be available as supplements, these were not available at time of data extraction. Other than one outcome reported in the registry but not the article (physical activity), there were no substantive differences between the pre-print article and the trial registry in procedures, population, treatments and outcomes.
The study was updated on March 25th, 2021 with data from Jama. |
Trial NCT04459247
Publication SHADE - Rastogi A, Postgrad Med J (2020) (published paper)
Funding: No specific funding (None)
Conflict of interest: No
| Methods | |
| RCT Blinding: Double blinded, no restrictions | |
| Location :
Single center / India Follow-up duration (days): 21 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Cholecalciferol 60 000 IU orally once a day for 7 days, afterwards once a week or once a day for another 7 days (if 25 (OH)D level>50 ng/ml). |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Cholecalciferol =16 Placebo=24 | |
| Characteristics of participants N= 40 Mean age : NR 20 males Severity : Mild: n=40 / Moderate: n=0 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register Virus negativity [ Time Frame: 21 days ] | |
| In the report Proportions of participants who turn SARS-CoV-2 negative(confirmed twice at 24-hour inter val) before week 3 | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
* |
| General comment |
This study is pending contact with authors.
In addition to the published article, the trial registry was used in data extraction and assessment of risk of bias. There were some differences between trial registry and published article in terms of population inclusion criteria. There were no differences in terms of study procedures, treatments and outcomes. Patient selection, study procedures and treatments are poorly described in both the published article and trail registry. There is no information about co-interventions given to participants as part of standard care. The study achieved its target sample size. |