Peginterferon Lambda-1 vs Placebo (RCT)
Mild outpatients
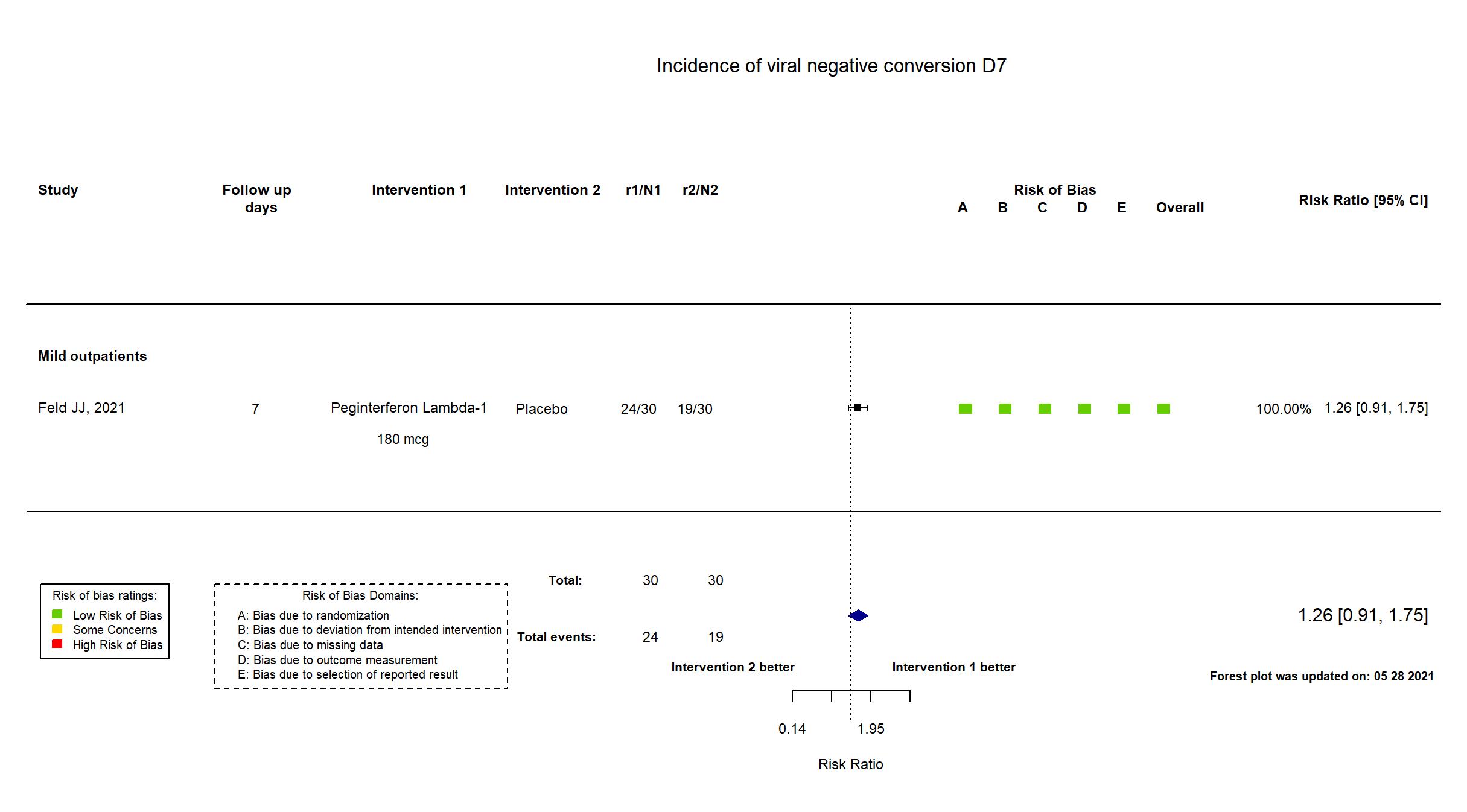
FOREST PLOTS -2022-10-07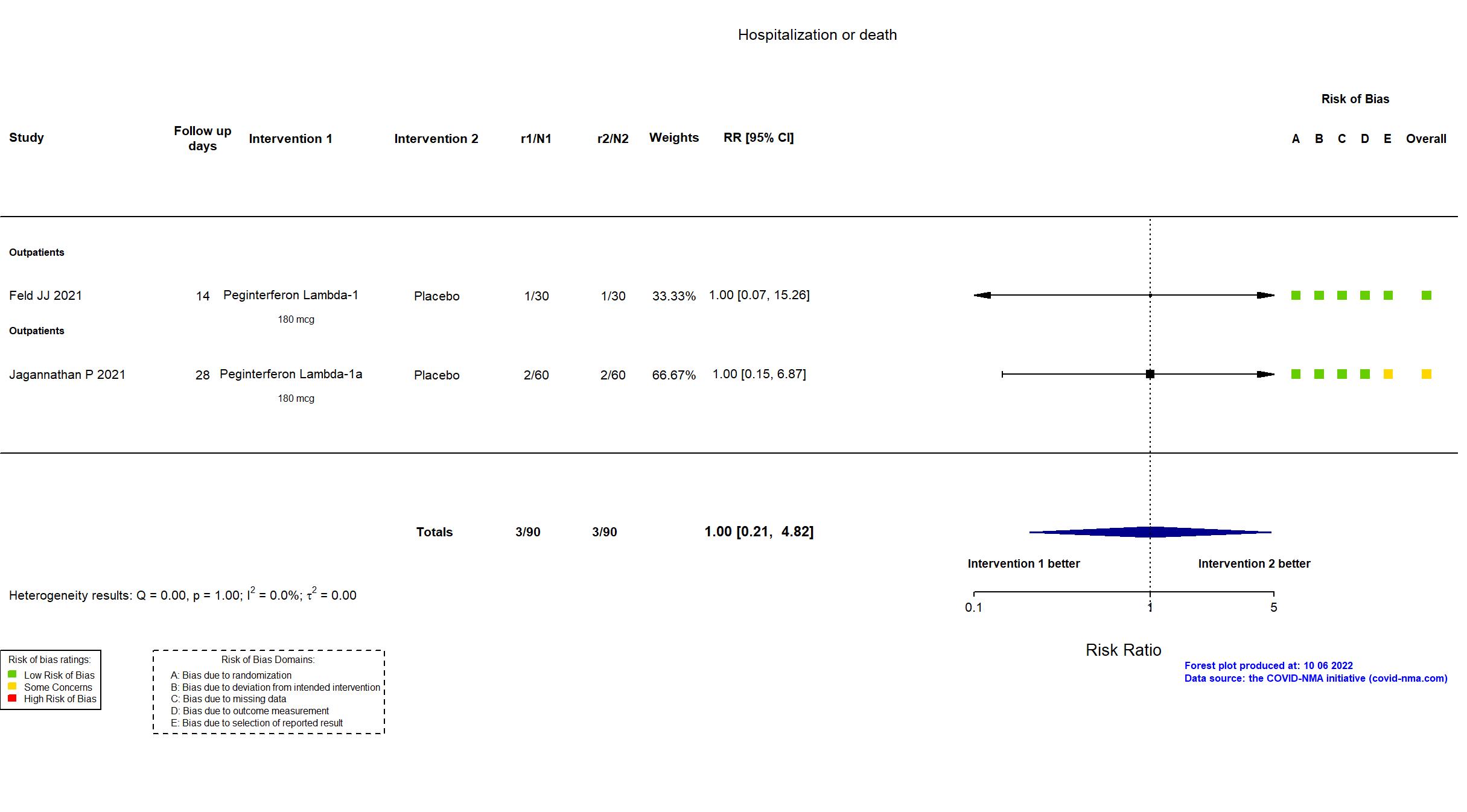
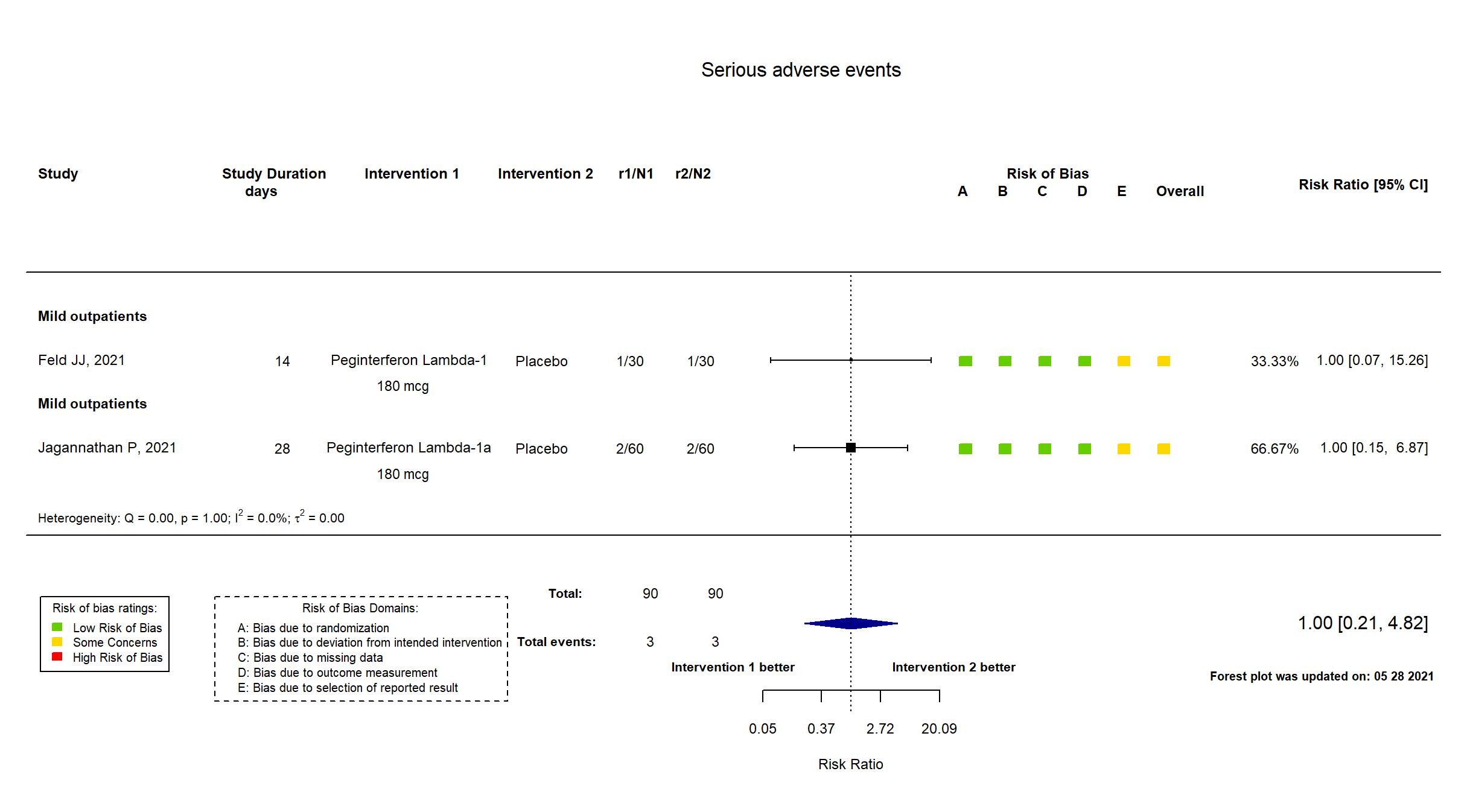







Trial NCT04354259
Publication ILIAD - Feld JJ, Lancet Respir Med (2021) (published paper)
Dates: 2020-05-18 to 2020-09-04
Funding: Mixed (Toronto COVID-19 Action Initiative; University of Toronto; Ontario First COVID-19 Rapid Research Fund; Eiger BioPharma (drug provision))
Conflict of interest: Yes
| Methods | |
| RCT Blinding: | |
| Location :
Multicenter / Canada Follow-up duration (days): 14 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Peginterferon Lambda-1 180 mcg subcutaneous injection once off. |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Peginterferon Lambda-1=30 Placebo=30 | |
| Characteristics of participants N= 60 Mean age : NR 25 males Severity : Mild: n= 49/ Asymptomatic: n=11 | |
| Primary outcome | |
| In the register Proportion swab negative at day 7 (Primary efficacy endpoint) (proportion of participants with negative SARS-CoV-2 RNA on nasopharyngeal swab); Treatment-emergent and treatment related serious adverse events (Primary Safety Endpoint) [Time Frame | |
| In the report Proportion of individuals with a negative MT swab for SARS-CoV-2 at Day 7; Incidence of treatment-emergent severe adverse events (SAEs) by Day 14 | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to all available versions of the published manuscript/pre-print article, the trial registry and supplementary materials were used in data extraction and assessment of risk of bias. The study protocol and statistical analysis plan were not available but were referenced in the article. There were some differences between the registry and pre-print in population. The registry required RT-PCR confirmed COVID-19 within 5 days of symptom onset whereas in the pre-print article asymptomatic patients with positive PCR within 7 days of recruitment were included. Negative conversion between a positive PCR within 7 days of recruitment likely accounts for 25% of patients having negative PCR results at baseline. The proportions of PCR-negative patients at baseline are not balanced between groups (17% intervention, 33% placebo). The report presents viral negative conversion results for those with confirmed PCR as a subgroup analysis (i.e., without randomisation). Some secondary outcomes in the registry were not reported. There were no other substantive differences in study procedures and interventions between the pre-print article and the registry. This study was updated on March 19th using data from the published manuscript. |
Trial NCT04331899
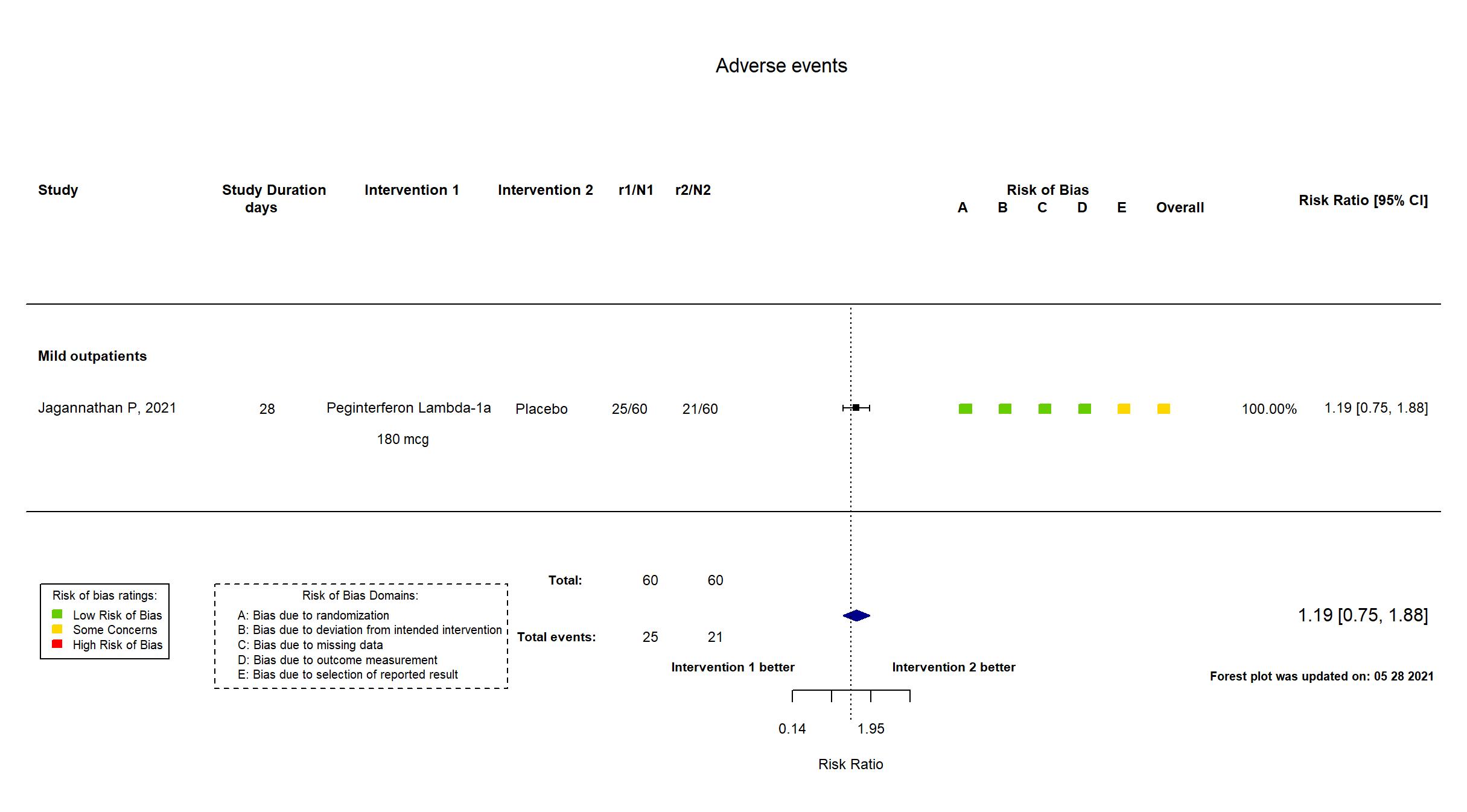
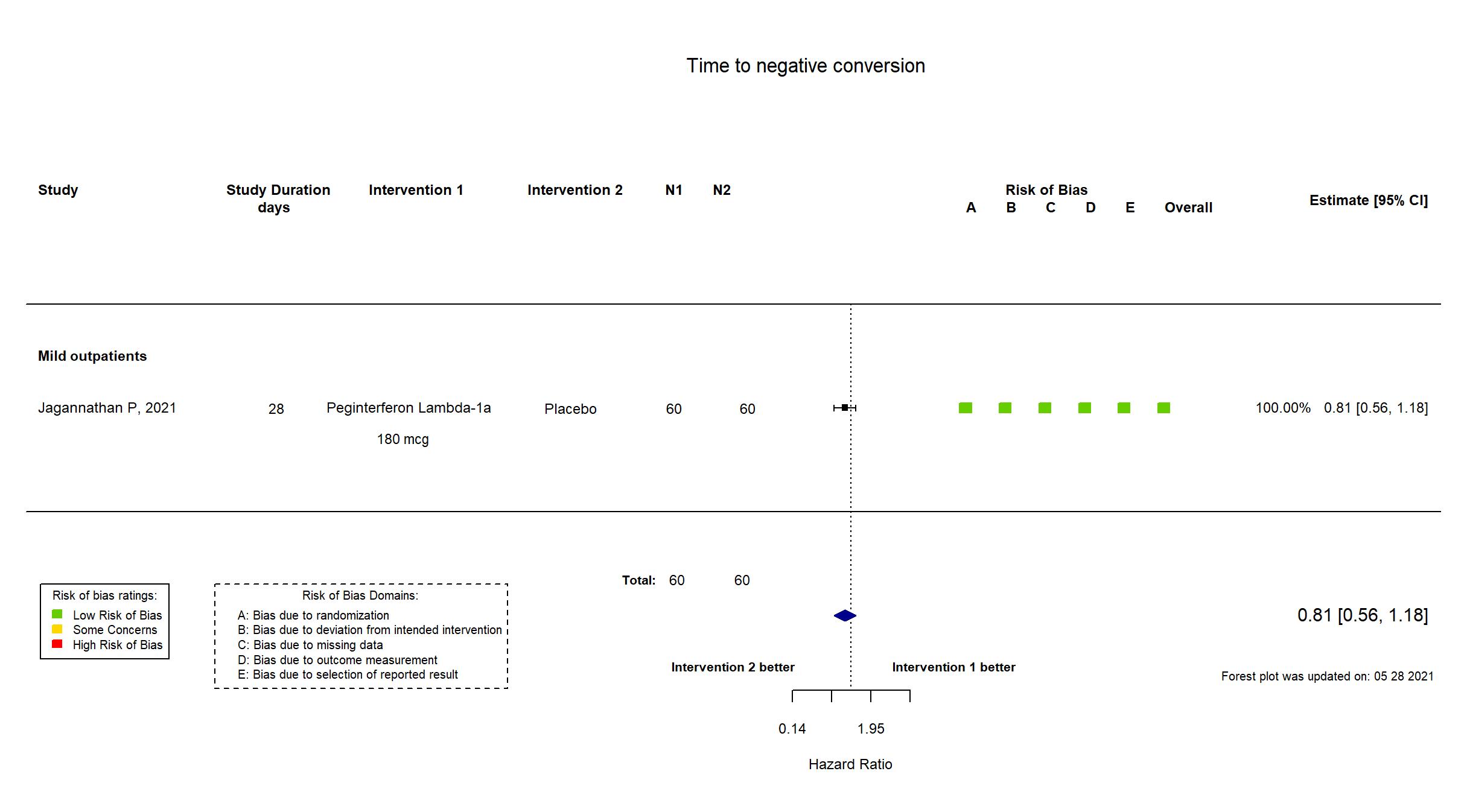
Publication Jagannathan P, Nature (2021) (published paper)
Dates: 2020-04-25 to 2020-07-17
Funding: Mixed (Anonymous donors to Stanford University; Eiger BioPharmaceuticals (drug))
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Single center / USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Peginterferon Lambda-1 180 mcg subcutaneous injection once off |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Peginterferon Lambda-1=60 Placebo=60 | |
| Characteristics of participants N= 120 Mean age : NR 70 males Severity : Mild: n= 112/ Asymptomatic: n=8 | |
| Primary outcome | |
| In the register Duration of Viral shedding of SARS-CoV-2 by qRT-PCR [ Time Frame: 28 days ] Time to first of two consecutive negative respiratory secretions obtained by oropharyngeal and/or anterior nare swabs for SARS-CoV-2 by qRT-PCR | |
| In the report Time to first of two consecutive negative oropharyngeal tests for SARS-CoV-2 by RT-PCR | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the preprint article, the protocol, SAP, the study registry and supplementary appendix were used in data extraction and risk of bias assessment. The study achieved the target sample size specified in the trial registry. There is no change from the trial registration in the intervention and control treatments. The primary outcome indicated in the trial registry reflects the primary outcome reported in the paper. All secondary outcomes were added to the trial registration after completion of study recruitment and follow up, and the design was changed from open-label to single-blind.
Quote: "The protocol was amended on June 16th, 2020 after 54 participants were enrolled but before results were available to include adults up to 75 years of age and eliminate exclusion criteria for low white blood cell and lymphocyte count." On 12th of April, 2021, this study was updated based on the published report. This study was updated on September 28th, 2022 with data extracted from the registry. |