Remdesivir vs Standard Care/Placebo (RCT)
Mild outpatients
Abd-Elsalam S, Am J Trop Med Hyg, 2022 has been retracted on September 15, 2022. The study is excluded from the analysis and grade assessment.
FOREST PLOTS -2022-11-04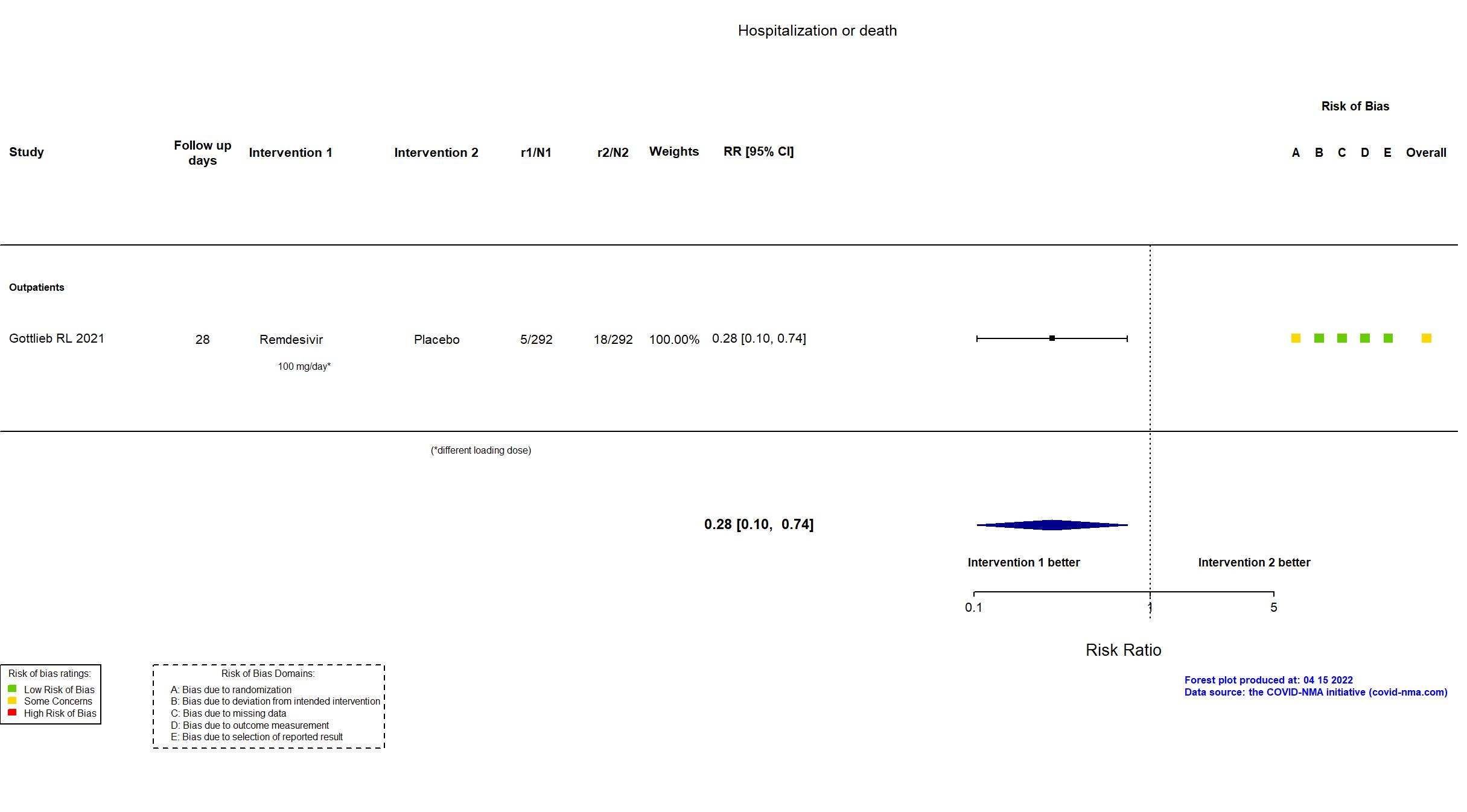
Abd-Elsalam S, Am J Trop Med Hyg, 2022 has been retracted on September 15, 2022. The study is excluded from the analysis and grade assessment.
FOREST PLOTS -2022-11-04




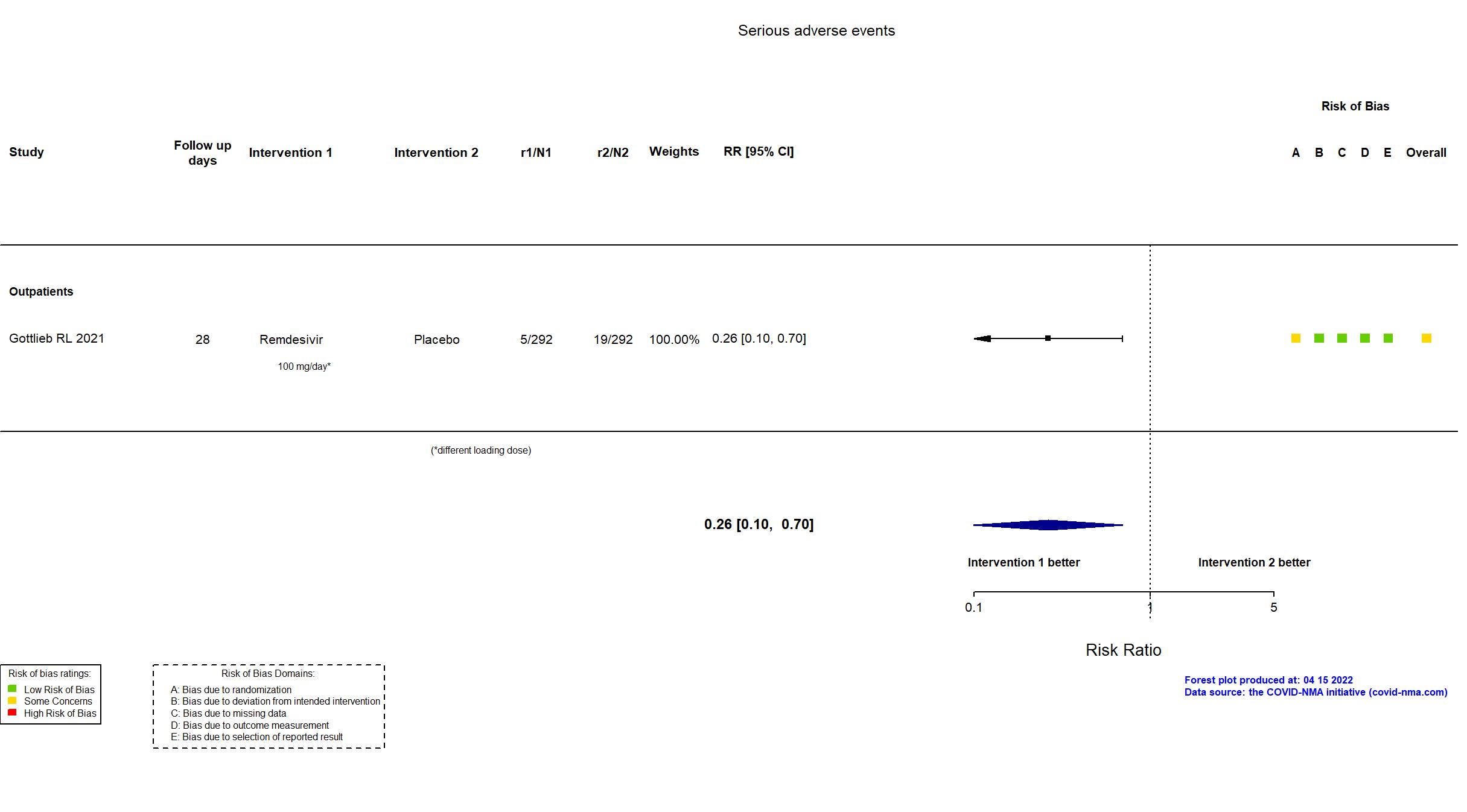
Trial NCT04501952; EudraCT 2020-003510-12.
Publication Gottlieb RL, N Engl J Med (2021) (published paper)
Dates: 2020-09-18 to 2021-04-08
Funding: Private (Gilead Sciences, Inc.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / Denmark, Spain, UK, USA Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Remdesivir Initial dose: 200 mg intravenously on day 1 - Maintenance dose: 100 mg intravenously on days 2 & 3 |
|
| Control
Placebo | |
| Participants | |
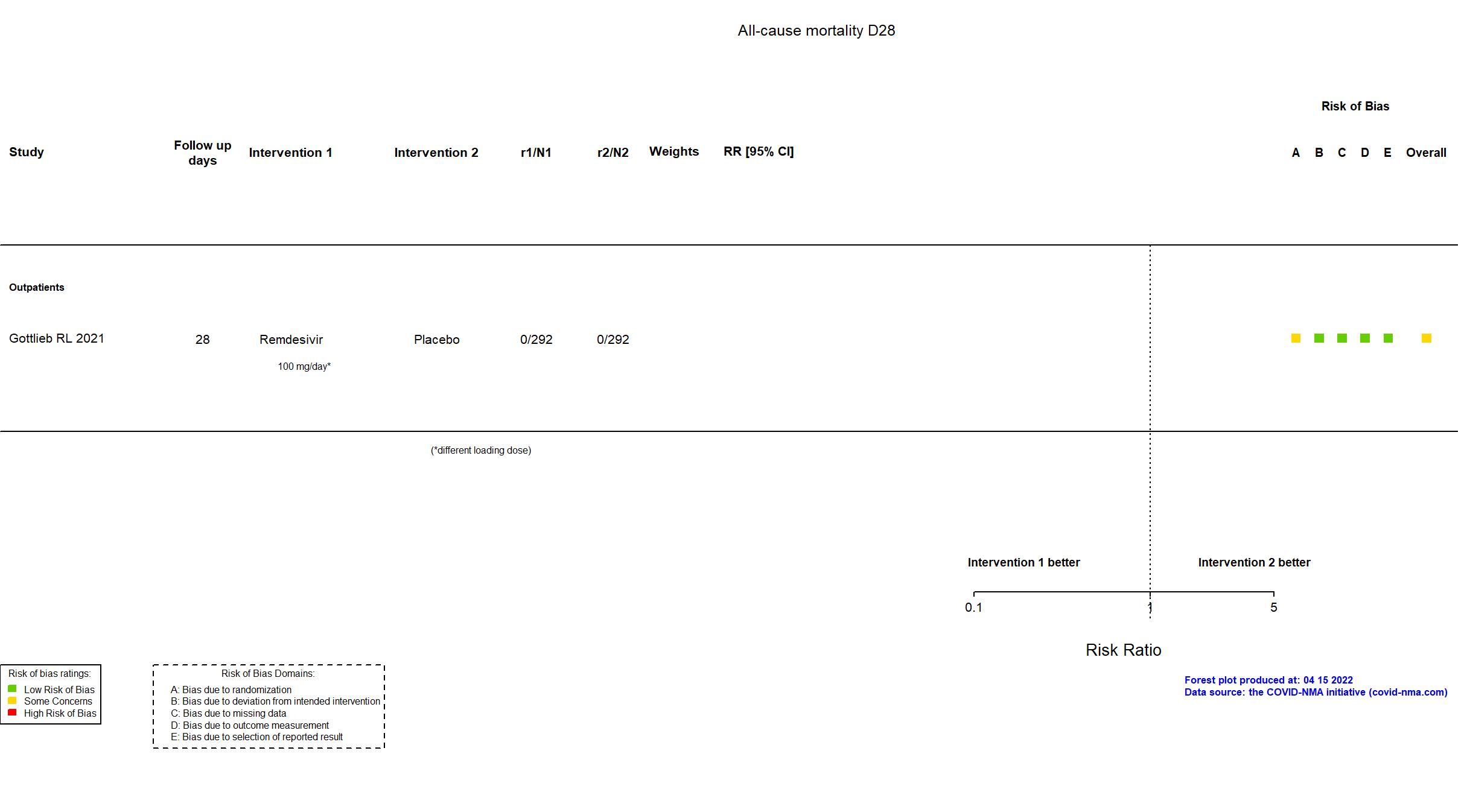
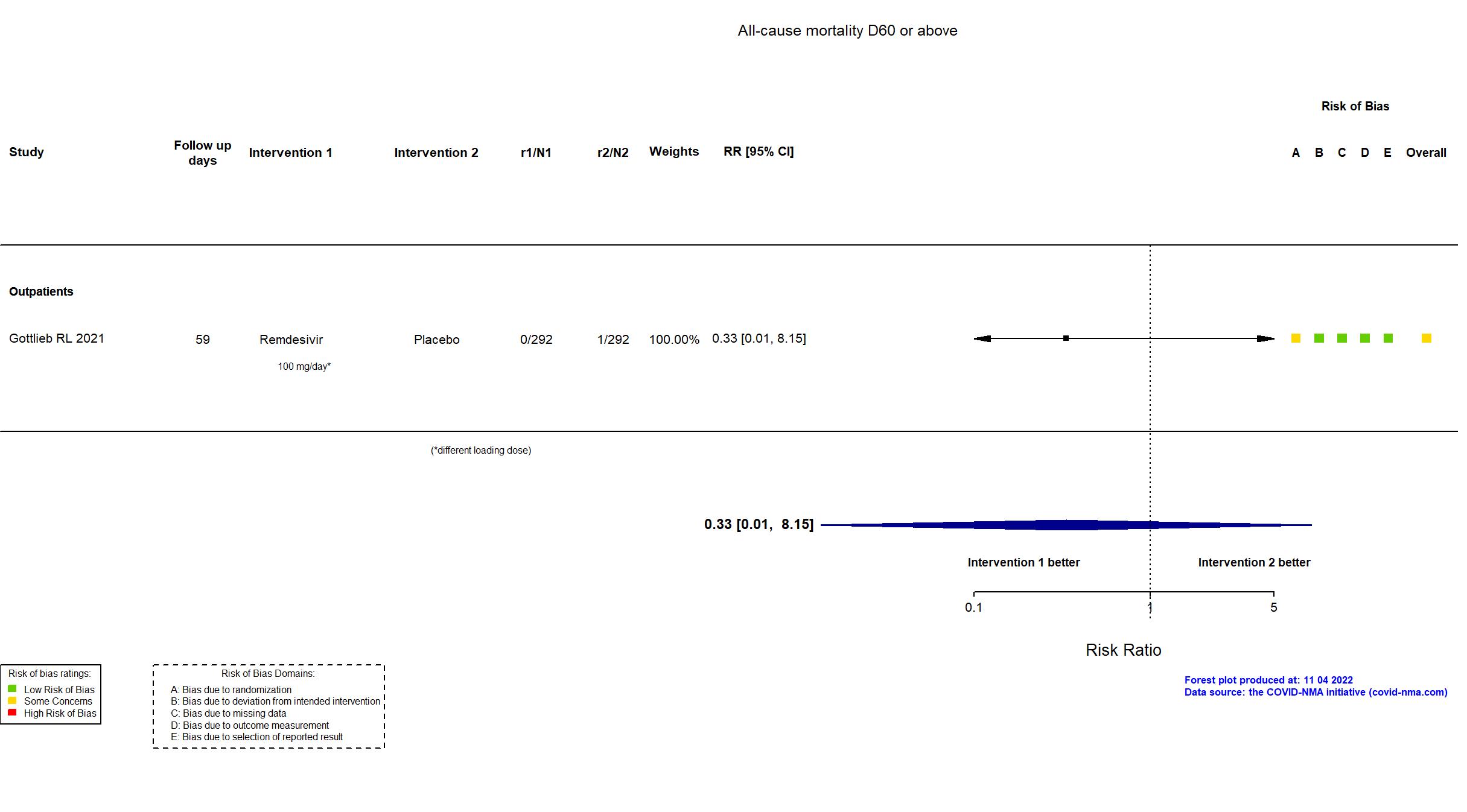
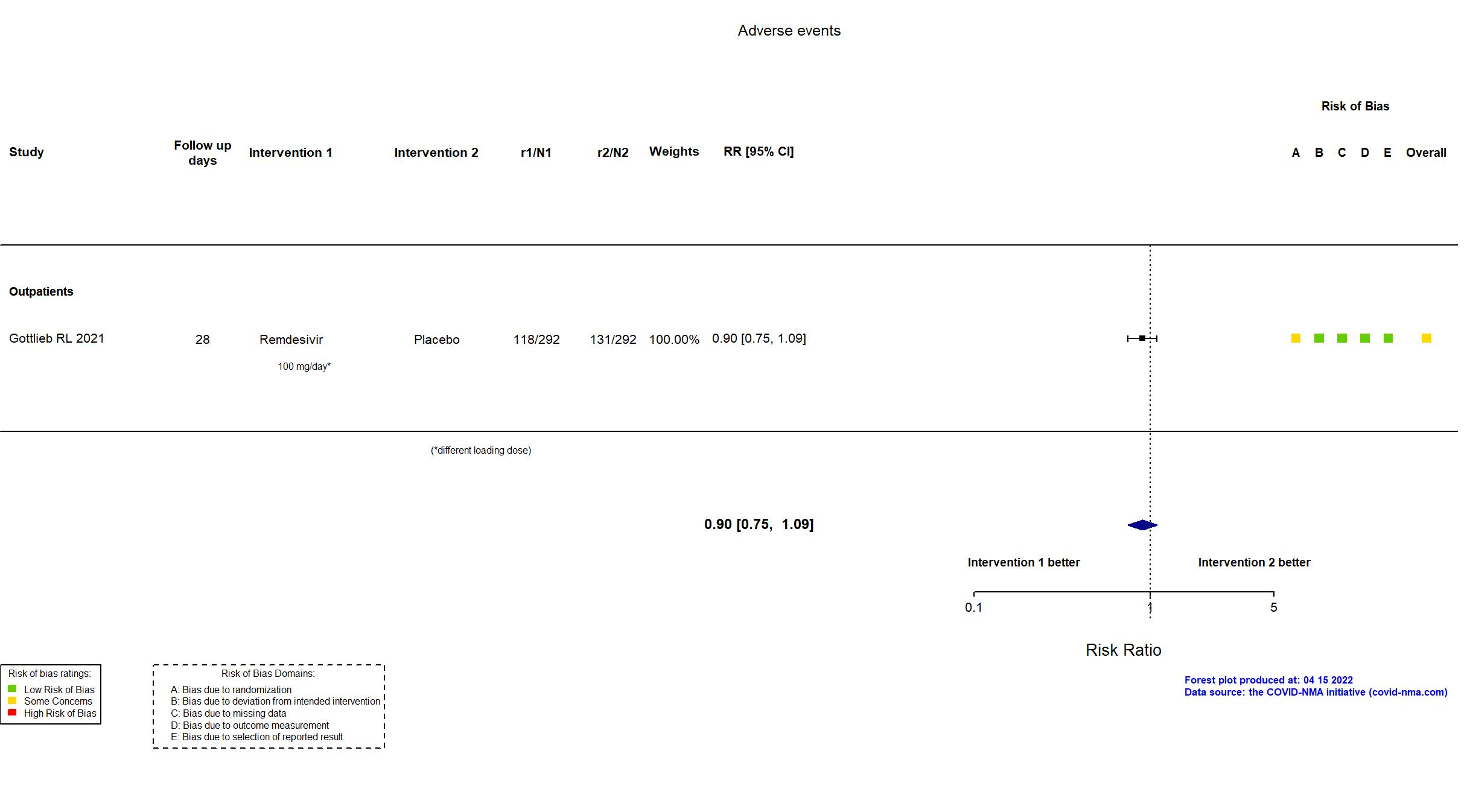
| Randomized participants : Remdesivir=292 Placebo=292 | |
| Characteristics of participants N= 584 Mean age : NR 293 males Severity : Mild: n= 584/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register 1) Percentage of Participants With Coronavirus Disease 2019 (COVID-19) Related Hospitalization (Defined as at Least 24 Hours of Acute Care) or All-Cause Death by Day 28 [ Time Frame: Randomization up to Day 28 ]; 2) Percentage of Participants Who Experienced Treatment-Emergent Adverse Events (TEAEs) [ Time Frame: First dose date up to last dose date (maximum: 3 days) plus 30 days ] | |
| In the report 1) A composite of hospitalization related to Covid-19 (as deter- mined by site investigators, who were unaware of trial-group assignments, and defined as ≥24 hours of acute care) or death from any cause by day 28; 2) adverse events. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, the prospective trial registries, protocol, statistical analysis plan and supplementary appendices were used in data extraction and assessment of risk of bias. The primary efficacy and safety outcomes in the article reflect those in the registry and protocol. Recruitment was terminated due to enrollment feasibility and changing needs of non-hospitalized participants and thus the trial (n = 584) did not achieve its target sample size (n = 1264).
This study was updated on November 3rd, 2022 with data extracted from the registry. |