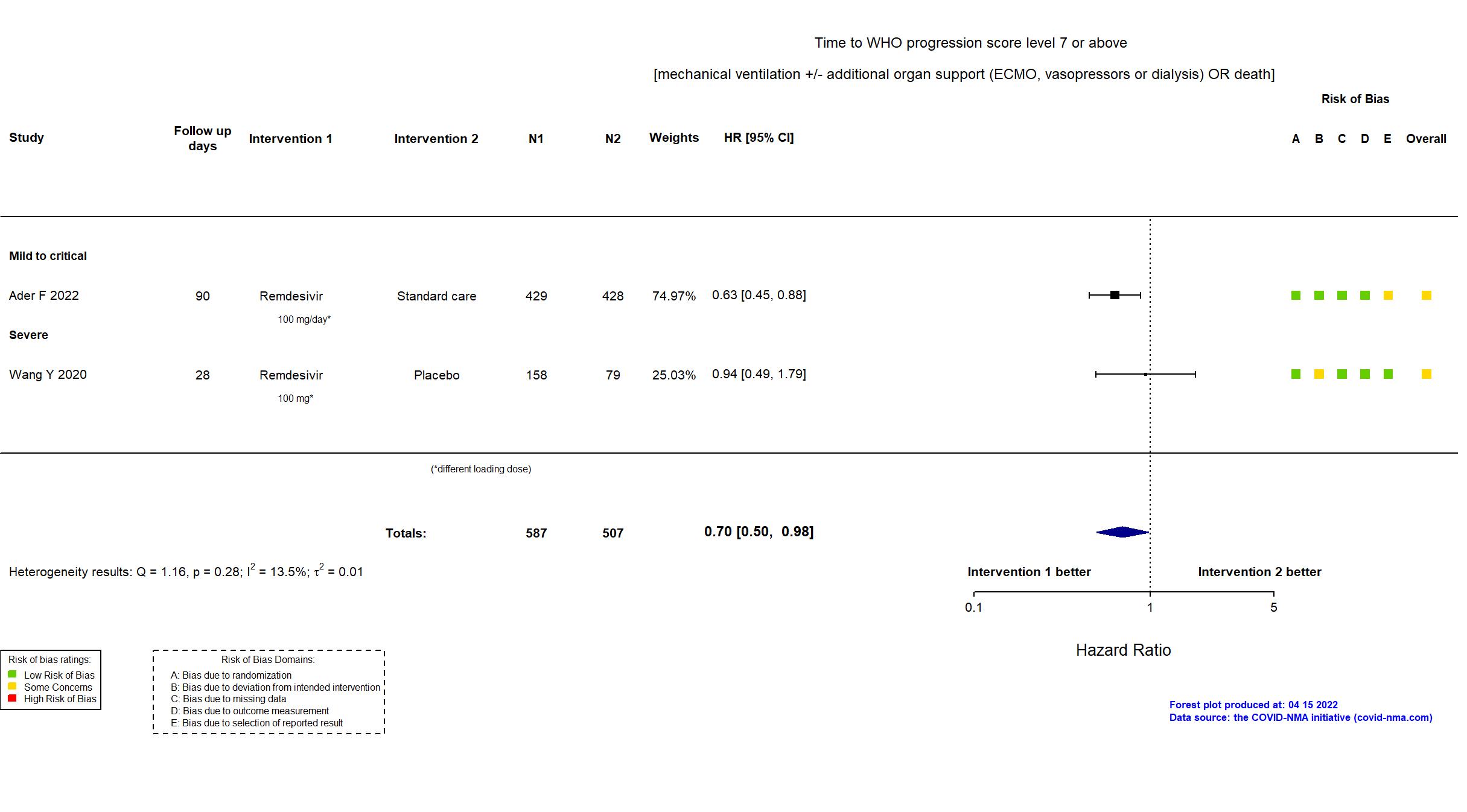
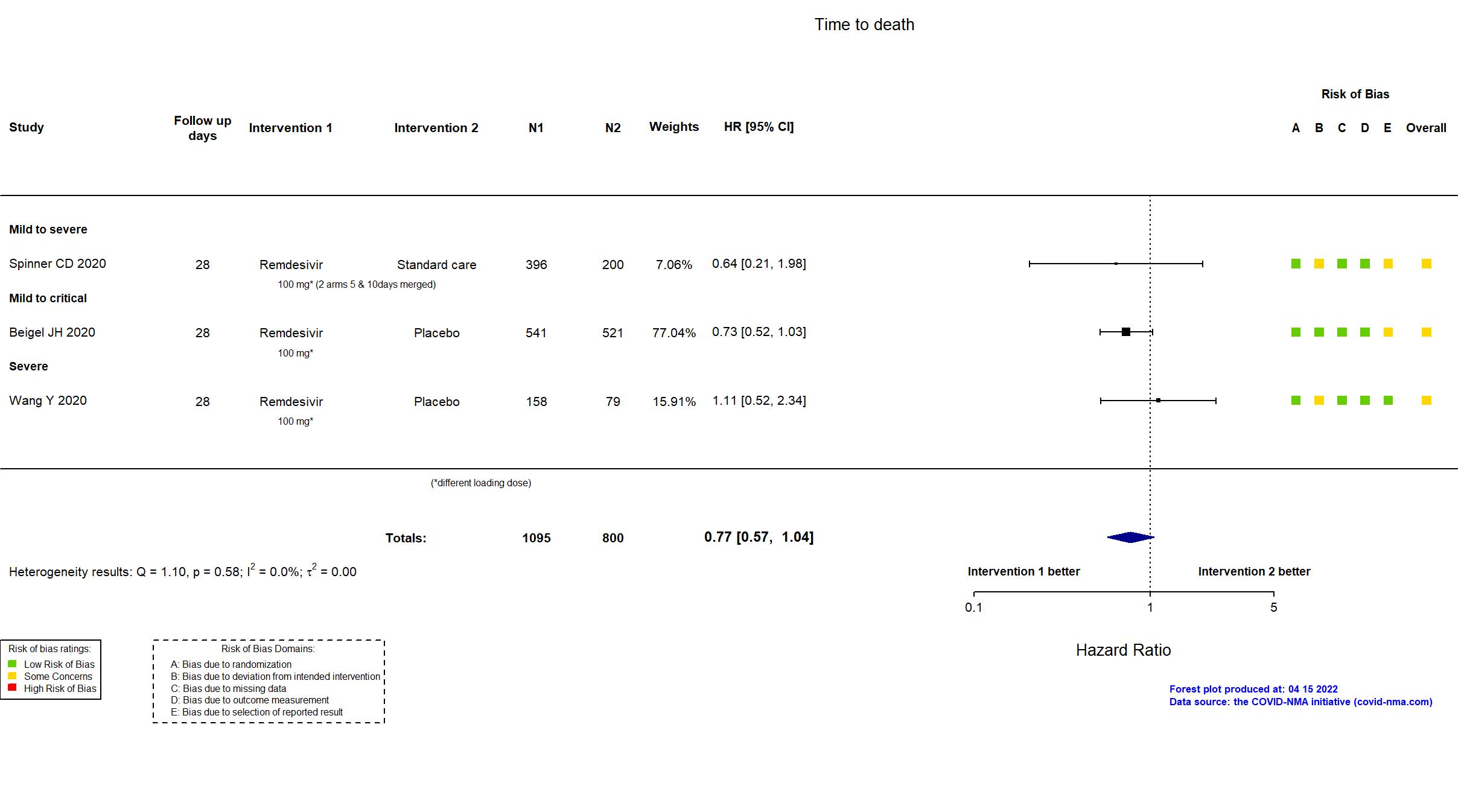
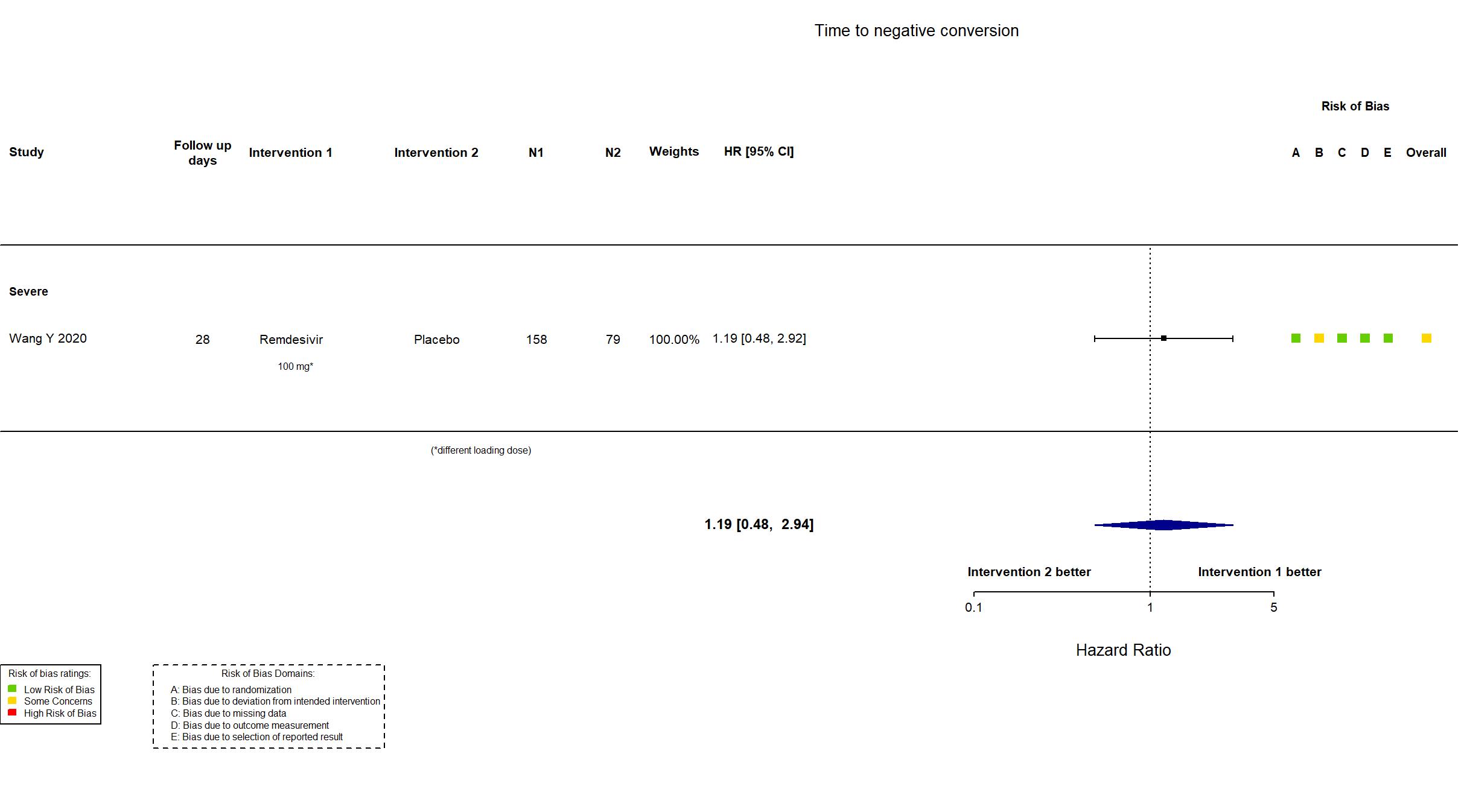
Remdesivir vs Standard Care/Placebo (RCT)
Hospitalized patients
Abd-Elsalam S, Am J Trop Med Hyg, 2022 has been retracted on September 15, 2022. The study is excluded from the analysis and grade assessment.
FOREST PLOTS -2023-02-23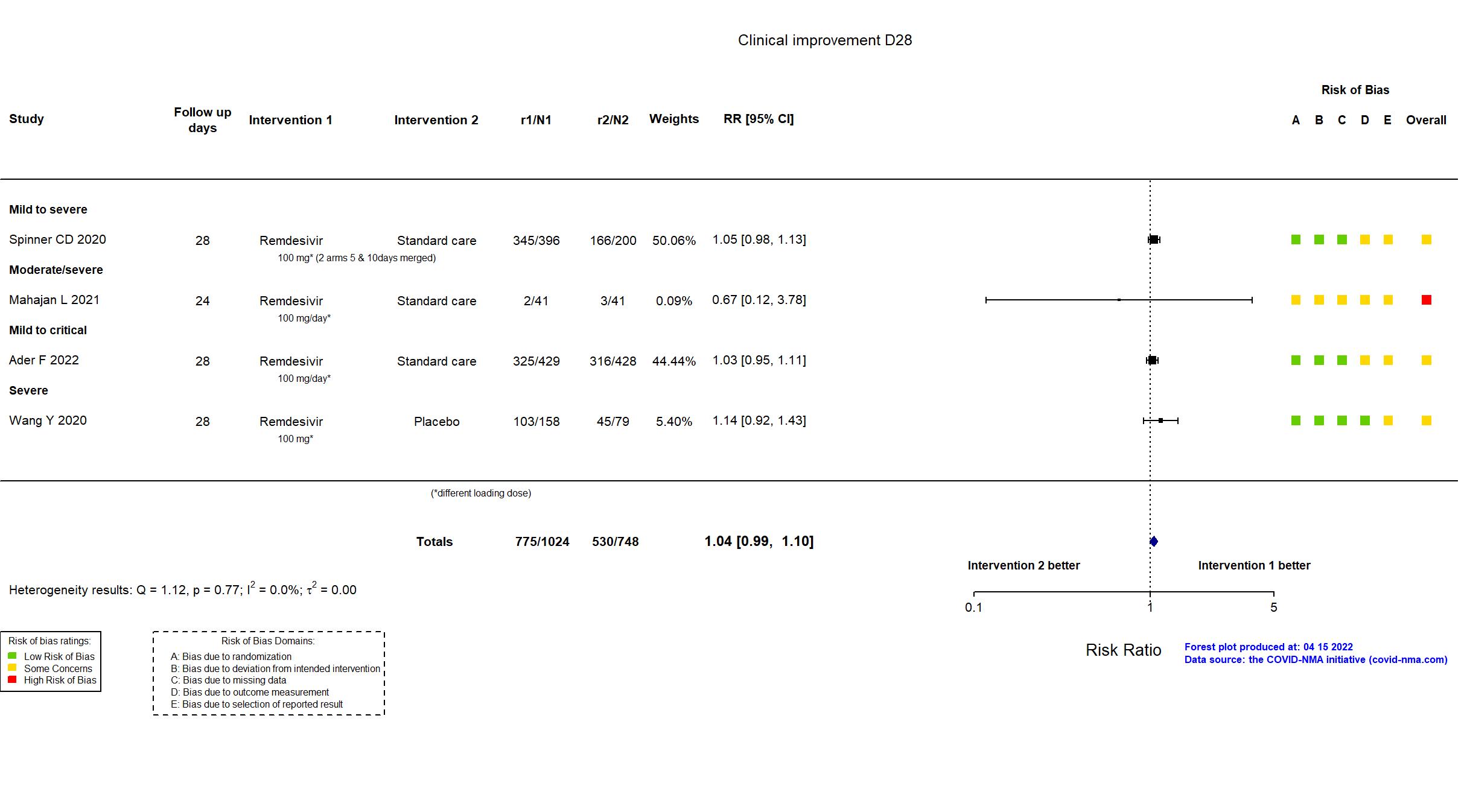
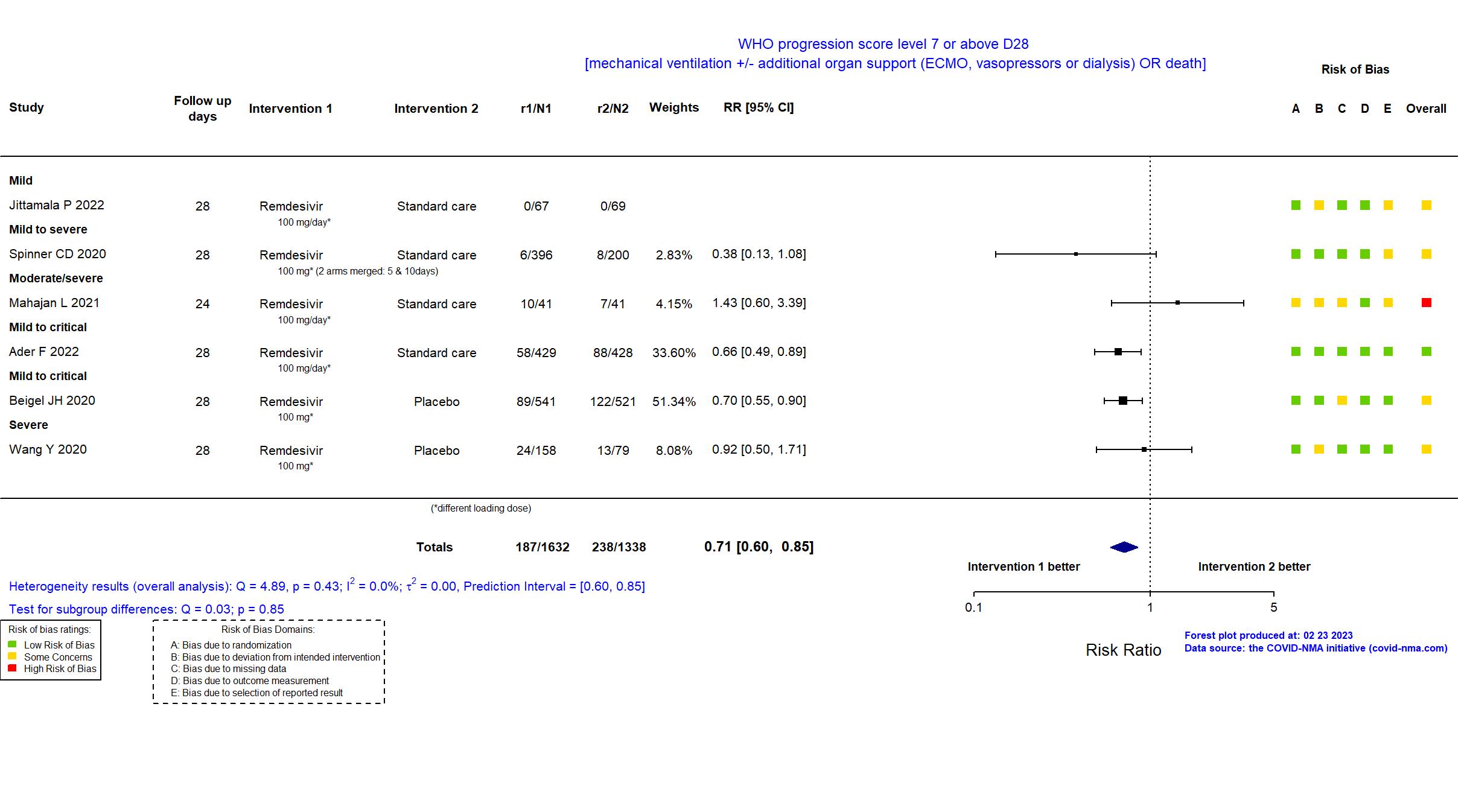
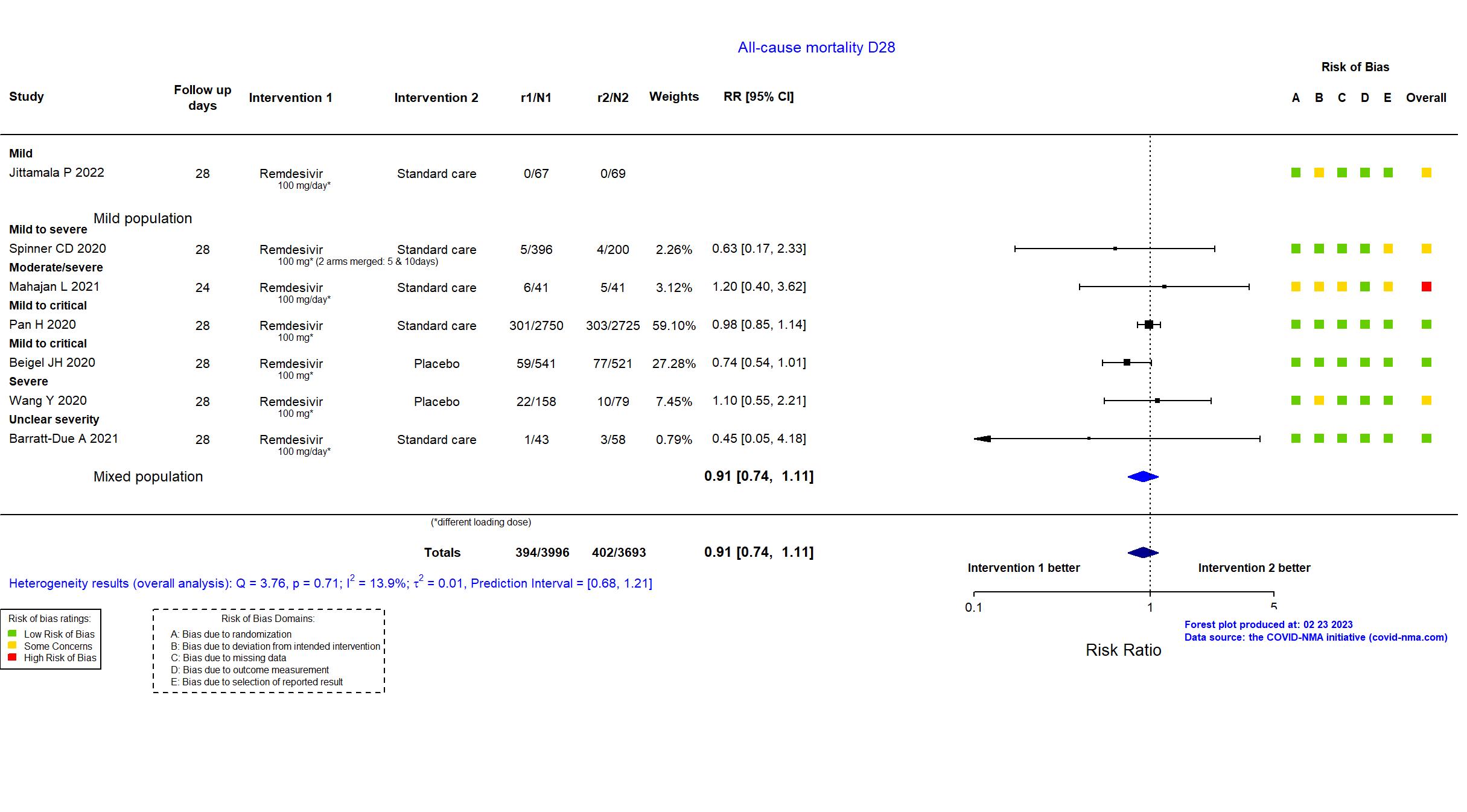
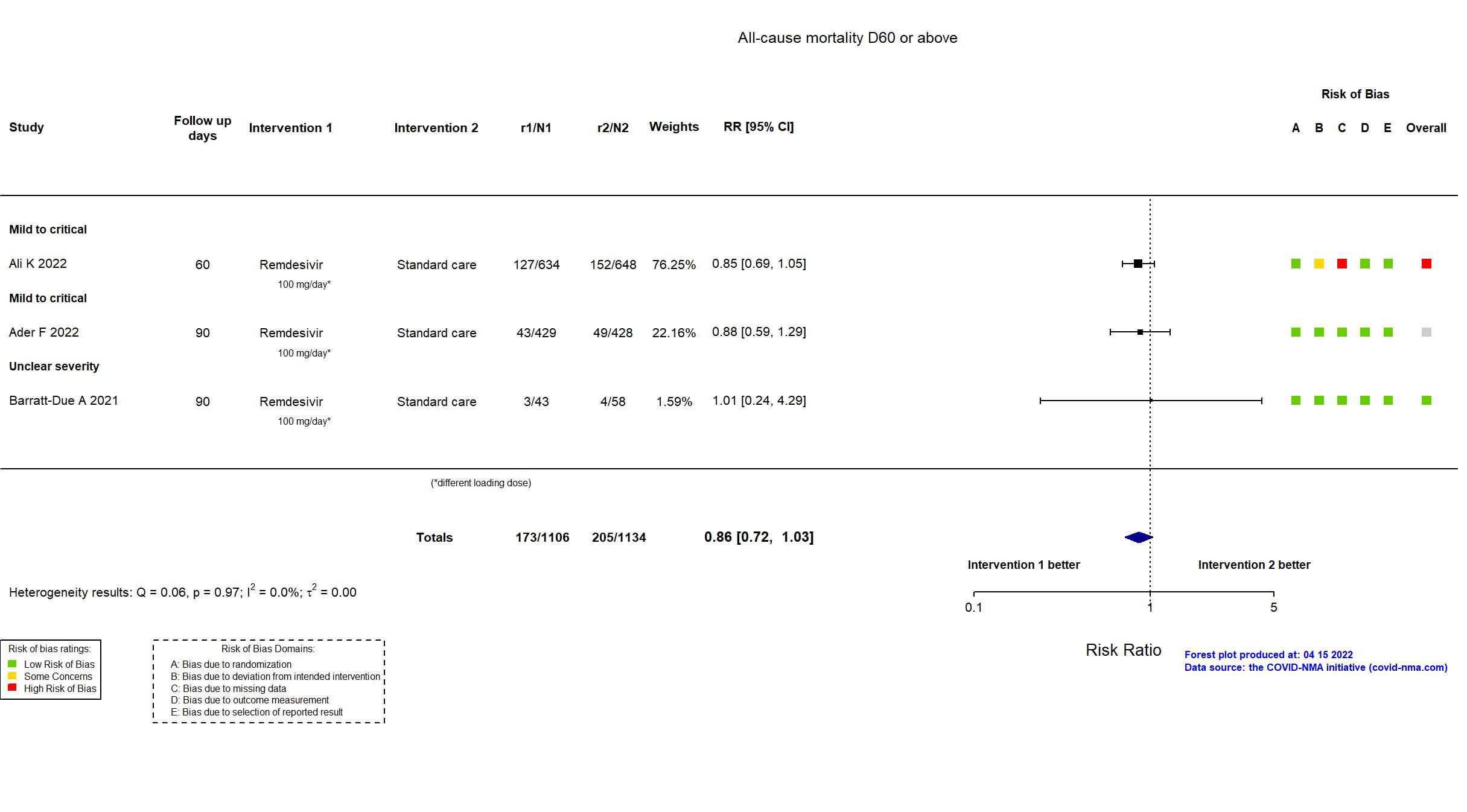
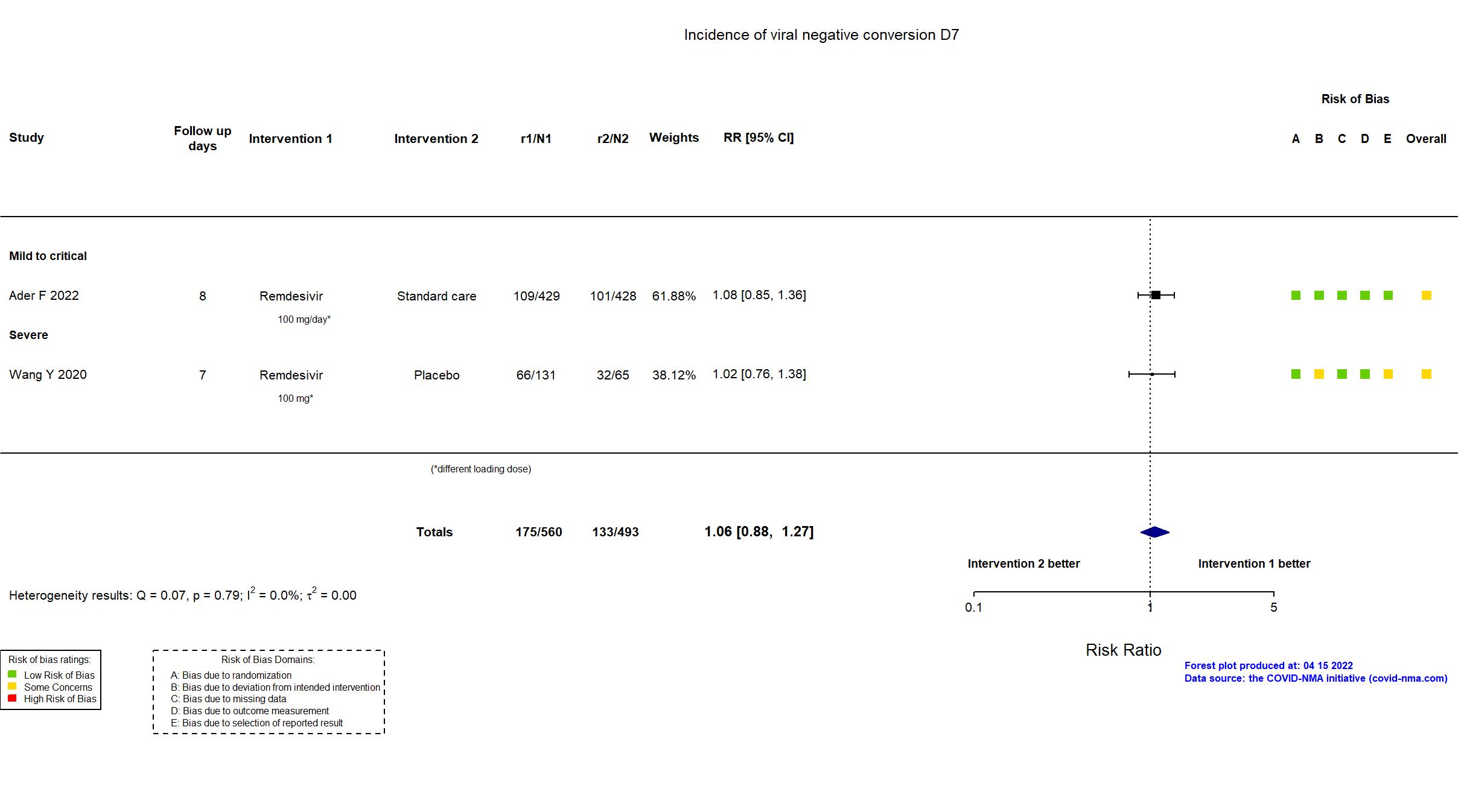
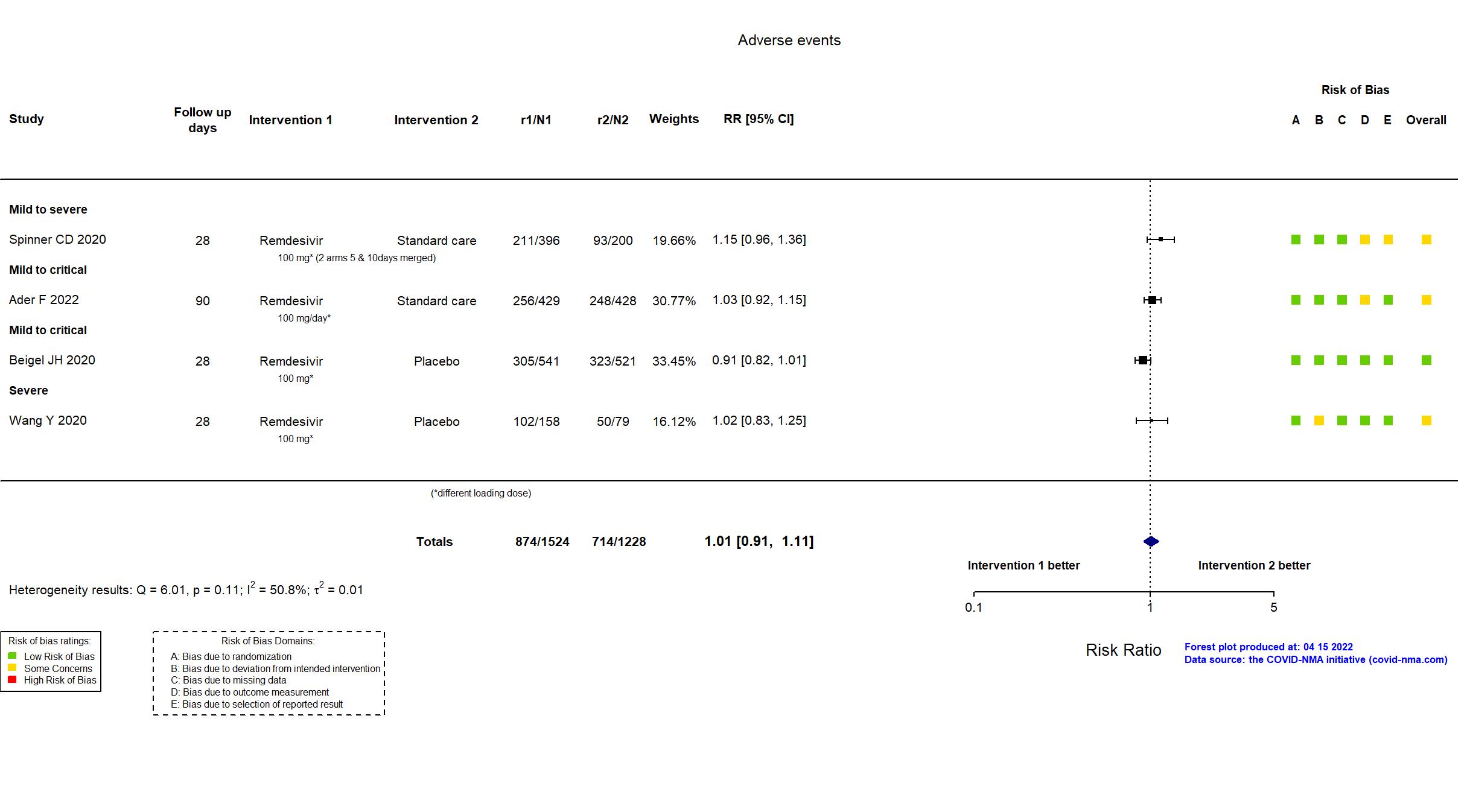
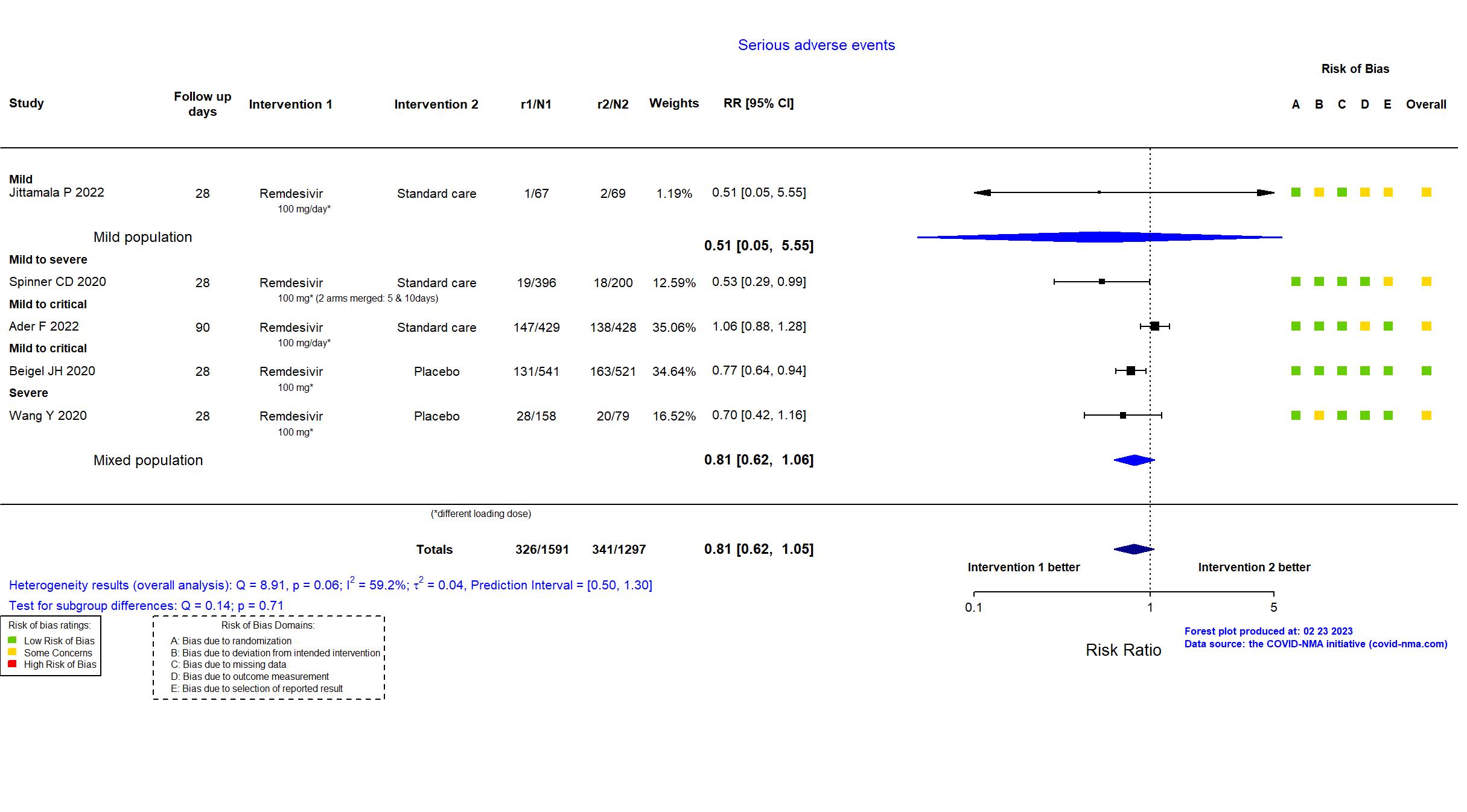
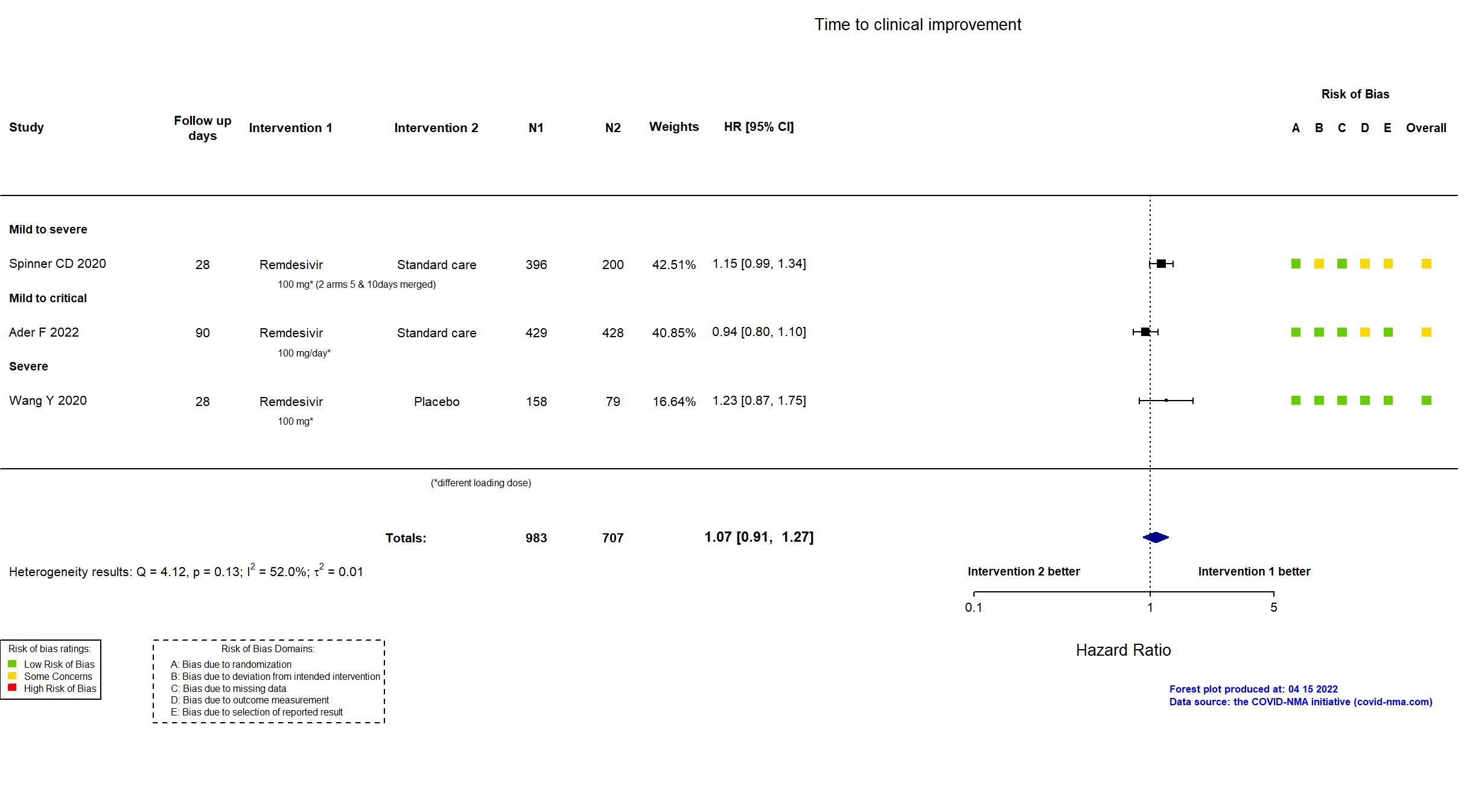
Abd-Elsalam S, Am J Trop Med Hyg, 2022 has been retracted on September 15, 2022. The study is excluded from the analysis and grade assessment.
FOREST PLOTS -2023-02-23










Trial NCT04315948, EudraCT2020-000936-23
Publication DisCoVeRy - Ader F, Lancet Infect Dis (2022) (published paper)
Dates: 2020-03-22 to 2021-01-21
Funding: Public/non profit (European Union Commission, French Ministry of Health, DIM One Health Île-de France, REACTing, Fonds Erasme-COVID-ULB, Belgian Health Care Knowledge Centre (KCE))
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Austria, Belgium, France, Luxembourg, Portugal Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Remdesivir Initial dose: 200 mg IV on the first day -Maintenance dose: 100 mg IV once a day for 5-9 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Standard care=428 Remdesivir=429 | |
| Characteristics of participants N= 857 Mean age : NR 588 males Severity : Mild: n=15 / Moderate: n=482 / Severe: n=192 Critical: n=154 | |
| Primary outcome | |
| In the register Percentage of subjects reporting each severity rating on a 7-point ordinal scale [ Time Frame: Day 15 ] | |
| In the report Clinical status at day 15 as measured on the 7-point ordinal scale of the WHO | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the pre-print/published article, the published protocol, supplementary appendices, prospective online trial registries and data gained from contact with authors were used in data analysis and risk of bias assessment. The pre-print reported on an interim analysis for the remdesivir intervention arm of a multiple-arm platform study (DisCoVeRy, an add-on trial to the WHO Solidarity consortium) that was terminated early due to futility. Consequently, the planned sample size was not achieved and long-term outcomes were not reported. Other than that, there were no substantial changes to procedures, population, or interventions. The outcome WHO Score 7 or above includes participants with events within 28 days rather than at 28 days. Mortality at day 28 was reported but not extracted due to overlapping patient population with Pan, N Engl J Med, 2020.
On 12th of July, 2021, this study was updated based on updated pre-print. On 6th of October, 2021, this study was updated based on the published article. On 16th of November, 2021, this study was updated based on data from contact with authors. On 14th of April, 2022, this study was updated based on the preprint reporting on the final results. On the 18th of November, 2022, this study was updated with the final results extracted from the publication. |
Trial NCT04330690
Publication Ali K, CMAJ (2022) (published paper)
Dates: 2020-08-14 to 2021-04-01
Funding: Public/non profit (Canadian Institutes of Health Research, the Vancouver Coastal Health Research Institute, the Northern Alberta Clinical Trials and Research Centre, Covenant Health Research Centre, the McGill Interdisciplinary Initiative in Infection and Immunity, the St. Joseph’s Health Care Foundation, and the London Health Sciences Foundation)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Canada Follow-up duration (days): 60 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Remdesivir Initial dose: 200 mg intravenously on day 0 - Maintenance dose: 100 mg intravenously on days 1 through 9. |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Remdesivir=634 Standard care=648 | |
| Characteristics of participants N= 1282 Mean age : NR 766 males Severity : Mild: n=125 / Moderate: n=697 / Severe: n=347 Critical: n=112 | |
| Primary outcome | |
| In the register All-cause mortality [Time Frame: 29 days] | |
| In the report In-hospital mortality | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
High |
| General comment | “In addition to the published article, the supplemental appendix and study registry were used in data extraction and risk of bias assessment. Neither the protocol nor the statistical analysis plan was available. There is no change from the trial registration in the remdesivir and control treatments. The registry primary outcome is different from the reported primary outcome (all-cause mortality vs in-hospital mortality [Time Frame: 29 days].The estimated sample size (n=2900 participants) specified in the trial registry corresponds to a 3-arms in a 1:1:1 ratio randomization to either the control arm, consisting of standard supportive care treatment for COVID-19, or remdesivir plus standard supportive care or Interferon-beta-1a plus standard supportive care." |
Trial NCT04321616
Publication NOR-SOLIDARITY - Barratt-Due A, Ann Intern Med (2021) (published paper)
Dates: 2020-04-07 to 2020-10-04
Funding: Mixed (National Clinical Therapy Research in the Specialist Health Services, Norway; Gilead Sciences (drug donation))
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Norway Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Remdesivir Initial dose: 200 mg/day IV- Maintenance dose: 100 mg/day for up to 9 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Remdesivir=43 Standard care=58 | |
| Characteristics of participants N= 101 Mean age : NR 72 males Severity : Mild: n=* / Moderate: n=* / Severe: n=* Critical: n=* | |
| Primary outcome | |
| In the register All cause in-hospital mortality [Time Frame: 3 weeks] | |
| In the report In-hospital mortality (i.e. death during the original hospitalization; follow-up ceased at discharge), regardless of whether death occurred before or after day 28 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment |
In addition to the peer-reviewed journal and pre-print articles, the study registry and protocol were used in data extraction and risk of bias assessment. NOR-Solidarity includes additional data collected beyond the WHO Solidarity core follow-up. Whereas the remdesivir arm was continued in the WHO Solidarity trial, it was stopped in the NOR-Solidarity study, on October 5th due to 1) general low mortality in hospitalized patients in Norway, 2) the potential for untoward effects in ventilated patients, and 3) potentially little, if any, effect of remdesivir for patients with mild disease. Some outcomes were reported at a different follow-up point than pre-specified in the registry. In-hospital mortality is reported.
On 27th of July, 2021, this study was updated based on the published article. |
Trial NCT04280705
Publication Beigel JH, N Engl J Med (2020) (published paper)
Dates: 21feb2020 to 2020-04-20
Funding: Mixed (National Institute of Allergy and Infectious Diseases and others)
Conflict of interest: No
| Methods | |
| RCT Blinding: | |
| Location :
Multicenter / USA, Denmark, UK, Greece, Germany, Korea, Mexico, Spain, Japan, Singapore Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Remdesivir 200 mg IV on day 1, followed by 100 mg IV daily for up to 9 additional days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Remdesivir=541 Placebo=521 | |
| Characteristics of participants N= 1062 Mean age : NR 684 males Severity : Mild: n=138 / Moderate: n=435 / Severe: n=193 Critical: n=285 | |
| Primary outcome | |
| In the register Time to recovery [ Time Frame: Day 1 through Day 29 ] | |
| In the report Time to recovery, defined as the first day, during the 28 days after enrollment, on which a patient satisfied categories 1, 2, or 3 on the eight-category ordinal scale | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to all available versions of the published/pre-print article, the study registry, protocol, and statistical analysis plan were used in data extraction and risk of bias assessment.
The final report was published in the New England Journal of Medicine on October 8, 2020 and the data (for 29 day follow-up). Data were also posted on the registry website, clinicaltrials.gov on September 25th, 2020 which contained data through day 29 of follow-up. The original report, published on May 22, 2020, presented preliminary results up to day 14. After interim data analysis, the data and safety monitoring board recommended that the preliminary primary analysis report and mortality data be provided to trial team members from the National Institute of Allergy and Infectious Diseases (NIAID). These results were made public and were accessible to the treating physicians, who could request to be made aware of the treatment assignment of trial participants who had not completed day 29 if clinically indicated. Participants assigned to the placebo group could be given remdesivir. The study had a larger sample size than the target sample size specified in the trial registry (n=800). There was no change from the trial registration in the intervention and control treatments. The primary outcome measurement changed from the first version to the protocol, and is described in the second version of the protocol (both are provided in the article appendix). The authors provide an explanation for this decision, which took place before interim analysis, and was proposed by statisticians who had no knowledge of outcome data. This trial was updated on February 9th, 2021 after contact with authors. |
Trial NCT05041907
Publication PLATCOV - Jittamala P, medRxiv (2022) (preprint)
Dates: 2021-09-30 to 2022-06-10
Funding: Public/non profit (Wellcome Trust)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Thailand, Brazil Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Remdesivir Initial dose: 200 mg IV infusion on day 1 - Maintenance dose: 100 mg IV infusion daily days 2-5 |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Standard care=69 Remdesivir=67 | |
| Characteristics of participants N= 136 Mean age : NR 59 males Severity : Mild: n=136 / Moderate: n=0 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register Rate of viral clearance for newly available and repurposed drugs [ Time Frame: Days 0-7 ] ; Rate of viral clearance for positive controls (e.g. monoclonal antibodies) [ Time Frame: Days 0-7 ] ; Rate of viral clearance for small novel molecule drugs [ Time Frame: Days 0-7 ] | |
| In the report Viral clearance rate derived from the slope of the log10 oropharyngeal viral clearance curve over the first 7 days following randomization. The treatment effect is defined as the multiplicative change in viral clearance rate relative to the no study drug arm. | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the pre-print article, the trial registry, protocol, statistical analysis plan and supplementary appendices were used in data extraction and assessment of risk of bias. A target sample size was not pre-specified in this adaptive platform trial. There is no change from the trial registration in the intervention and control treatments. The registry primary outcome reflects the reported primary outcome. Total adverse events are not reported. |
Trial *
Publication Mahajan L, Indian J Anaesth (2021) (published paper)
Dates: 2020-06-01 to 2020-12-30
Funding: No specific funding (None)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / India Follow-up duration (days): 24 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Remdesivir Initial dose: 200 mg IV 3 to 4 times a day for the first 24 hours -Maintenance dose: 100 mg IV once daily for 4 days. |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Remdesivir=41 Standard care=41 | |
| Characteristics of participants N= 82 Mean age : NR 48 males Severity : Mild: n=0 / Moderate: n=53 / Severe: n=17 Critical: n=0 | |
| Primary outcome | |
| In the register NR | |
| In the report Improvement in clinical outcomes | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: Not reported |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
High |
| General comment | Only the published article was used in data extraction and assessment of the risk of bias. No trial registry, protocol or statistical analysis plan was available. The study sample size was reached. Outcome data assessed as per-protocol analysis. The study was assessed to be at a high risk of bias due to some concerns in four of five domains. |
Trial ISRCTN83971151; NCT04315948
Publication SOLIDARITY (RDV) - Pan H, N Engl J Med (2020) (published paper)
Dates: 2020-03-22 to 2020-10-04
Funding: Mixed (World Health Organization; Gilead Sciences (drug donation))
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Multinational (30) Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Remdesivir 200 mg IV infusion at day-0 followed by 100 mg once a day for next 9 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Remdesivir=2750 Standard care=2725 | |
| Characteristics of participants N= 5475 Mean age : NR 3431 males Severity : Mild: n=1325 / Moderate: n=* / Severe: n=* Critical: n=* | |
| Primary outcome | |
| In the register All-cause mortality, subdivided by the severity of disease at the time of randomization, measured using patient records throughout the study | |
| In the report In-hospital mortality | |
| Documents avalaible |
Protocol Yes. In English Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment |
In addition to all available versions of the published manuscript, the pre-print article, the study registry and protocol were used in data extraction and risk of bias assessment. This was a report of interim results of an adaptive trial evaluating 4 drugs: Remdesivir, Hydroxychloroquine, Lopinavir, and Interferon-β1a. No target sample size was pre-specified.
Quote: "The protocol stated “The larger the number entered the more accurate the results will be, but numbers entered will depend on how the epidemic develops… it may be possible to enter several thousand hospitalised patients with relatively mild disease and a few thousand with severe disease, but realistic, appropriate sample sizes could not be estimated at the start of the trial.” The Executive Group, blind to any findings, decided the timing of release of interim results." Quote: "The hydroxychloroquine, lopinavir, and interferon regimens were discontinued for futility on, respectively, June 19, July 4, and October 16, 2020." There was no change from the trial registration in the intervention and control treatments, nor in the primary and secondary outcomes. This study was updated on December 9th using data from the published manuscript. |
Trial NCT04292730
Publication Spinner CD, JAMA (2020) (published paper)
Dates: 2020-03-15 to 2020-04-18
Funding: Private (Gilead Sciences)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Multinational Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Remdesivir 200 mg IV infusion on day-1 followed by 100 mg once a day for next 9 days Remdesivir 10 days 200 mg IV infusion on day-1 followed by 100 mg once a day for next 9 days Remdesivir 5 days 200 mg IV infusion on day-1 followed by 100 mg once a day for next 4 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Remdesivir=396 Remdesivir 10 days=197 Remdesivir 5 days=199 Standard care=200 | |
| Characteristics of participants N= 992 Mean age : NR 475 males Severity : Mild: n=660 / Moderate: n=111 / Severe: n=6 Critical: n=0 | |
| Primary outcome | |
| In the register The Odds of Ratio for Improvement on a 7-point Ordinal Scale on Day 11 [ Time Frame: Day 11 ] The odds ratio represents the odds of improvement in the ordinal scale between the treatment groups. The ordinal scale is an assessment of the clinical stat | |
| In the report Clinical status assessed by a 7-point ordinal scale on Day 11 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the study registry, protocol, SAP and supplementary files were used in data extraction and risk of bias assessment. This is a report on a multinational 3 arm RCT in Phase A and Phase B is ongoing. Quote: "In addition, a non-randomized extension phase was added in which up to 1000 additional patients could be enrolled to receive remdesivir; the results of the extension phase will be the subject of a subsequent report". The target sample size specified in the registry 1113 was not yet achieved. There is no change from the trial registration in the intervention and control treatments. Secondary outcomes in the report are not specified in the trial registry however they are congruent with the protocol. Quote: "The protocol was amended on March 15, 2020, on the basis of emerging understanding of the clinical presentation and assessment of COVID-19. The age limit for eligibility was lowered from 18 years to 12 years and the minimum temperature requirement was eliminated. The primary end point in the original protocol was the proportion of patients discharged by day 14 of the study. This was amended to assessment of clinical status on a 7-point ordinal scale by day 11 |
Trial NCT04257656
Publication Wang Y, Lancet (2020) (published paper)
Dates: 06feb2020 to 12mar2020
Funding: Mixed (Chinese Academy of Medical Sciences Emergency Project of COVID-19, National Key Research and Development Program of China, the Beijing Science and Technology Project)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Participants, outcome assessor and health care pro | |
| Location :
Multicenter / China Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Remdesivir 200 mg IV infusion at day-1 followed by 100 mg IV infusion once a day for next 9 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Remdesivir=158 Placebo=79 | |
| Characteristics of participants N= 237 Mean age : NR 140 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=235 Critical: n=1 | |
| Primary outcome | |
| In the register Time to Clinical Improvement (TTCI) [Censored at Day 28] [ Time Frame: up to 28 days ] | |
| In the report Time to clinical improvement within 28 days after randomisation | |
| Documents avalaible |
Protocol Yes. In English Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the study registry and protocol were used in data extraction and risk of bias assessment. There was no a-priori statistical analysis plan. The study is underpowered, because recruitment was terminated early by the data safety and monitoring board, on the basis of the termination criteria specified in the protocol. With the actual number of participants (vs the target sample size), the statistical power was reduced from 80% to 58%. There is no change from the trial registration in the intervention and control treatments. The outcome adverse events is reported in the paper, but was not pre-specified in the trial registry/protocol. On July 15th 2020, we received additional information from authors on this study. |