Vilobelimab vs Placebo/Standard care (RCT)
Hospitalized patients
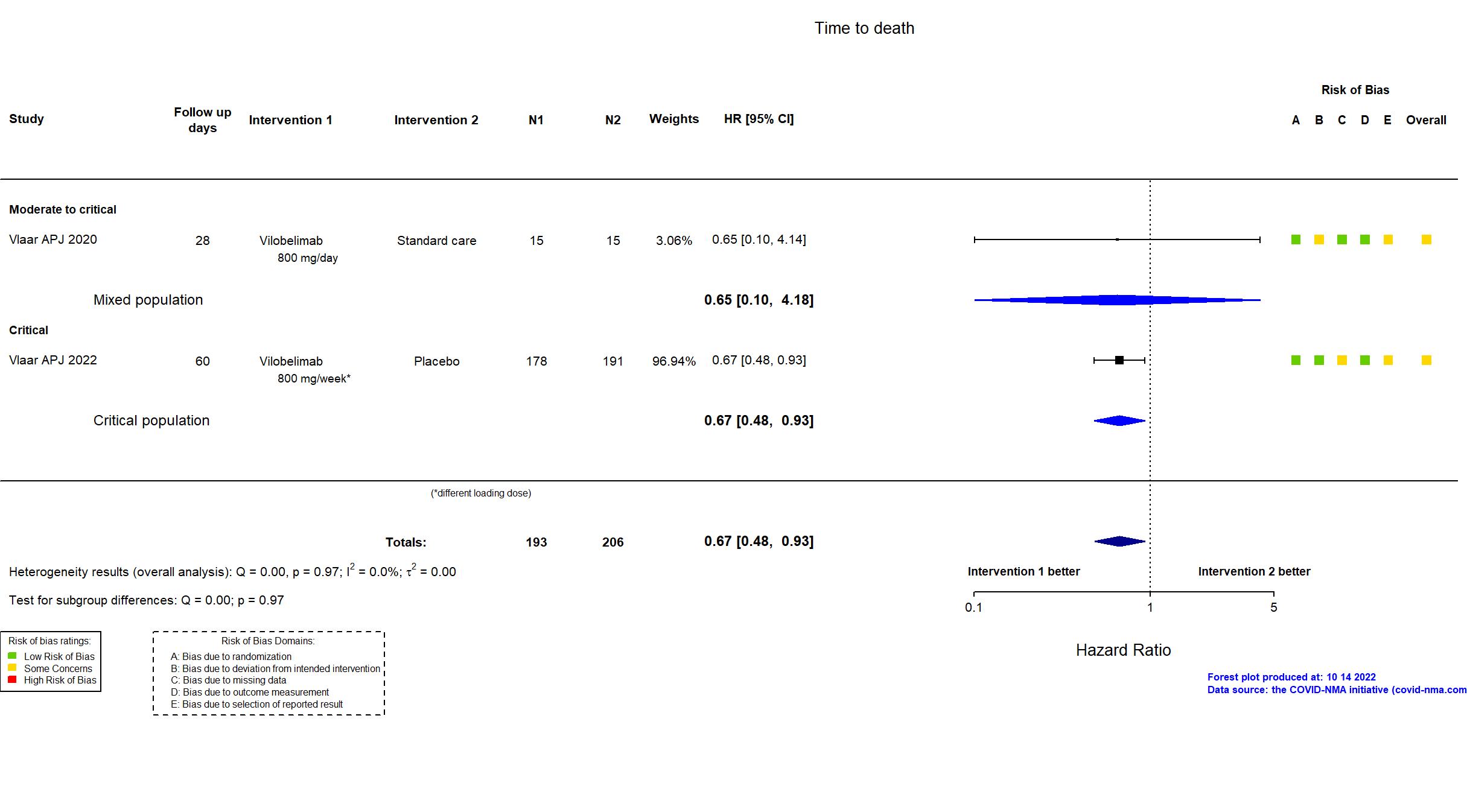
FOREST PLOTS -2022-10-14






Trial NCT04333420, EudraCT 2020-001335-28
Publication PANAMO - Vlaar APJ, Lancet Respir Med (2022) (published paper)
Dates: 2020-10-01 to 2021-10-04
Funding: Mixed (This trial was funded by InflaRx and partly funded by the German Federal Government. The funder of the study had a role in study design, data collection, data analysis, data interpretation, and writing of the report.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: triple blinding | |
| Location :
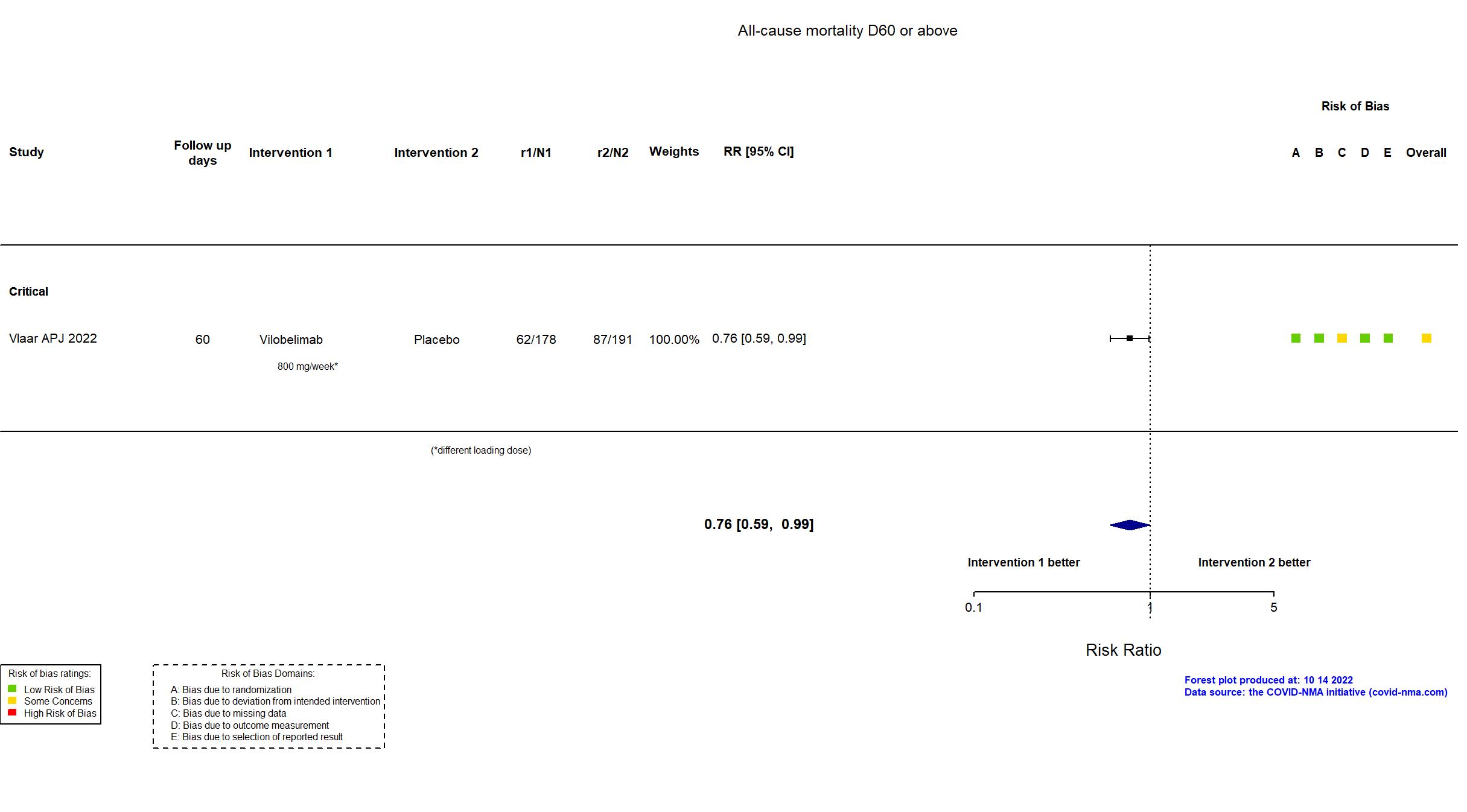
Multicenter / Netherlands (37%), Brazil (20%), Mexico (10%), France (9%), Russia (6%), Germany (6%), Belgium (4%), Peru (4%), and South Africa (3%) Follow-up duration (days): 60 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Vilobelimab 800 mg IV for a maximum of 6 doses, as one dose per day on days 1, 2, 4, 8, 15, and 22 |
|
| Control
Placebo | |
| Participants | |
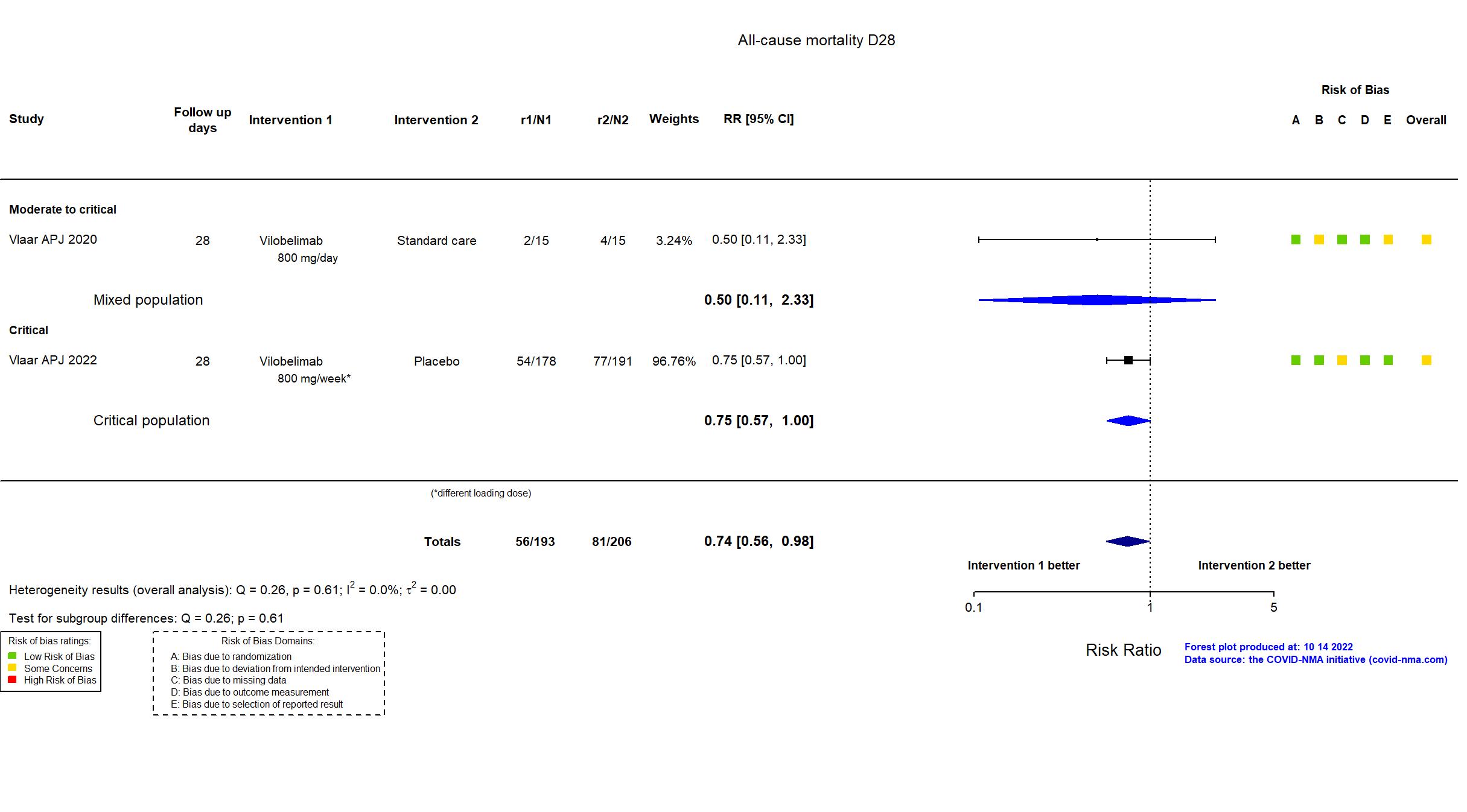
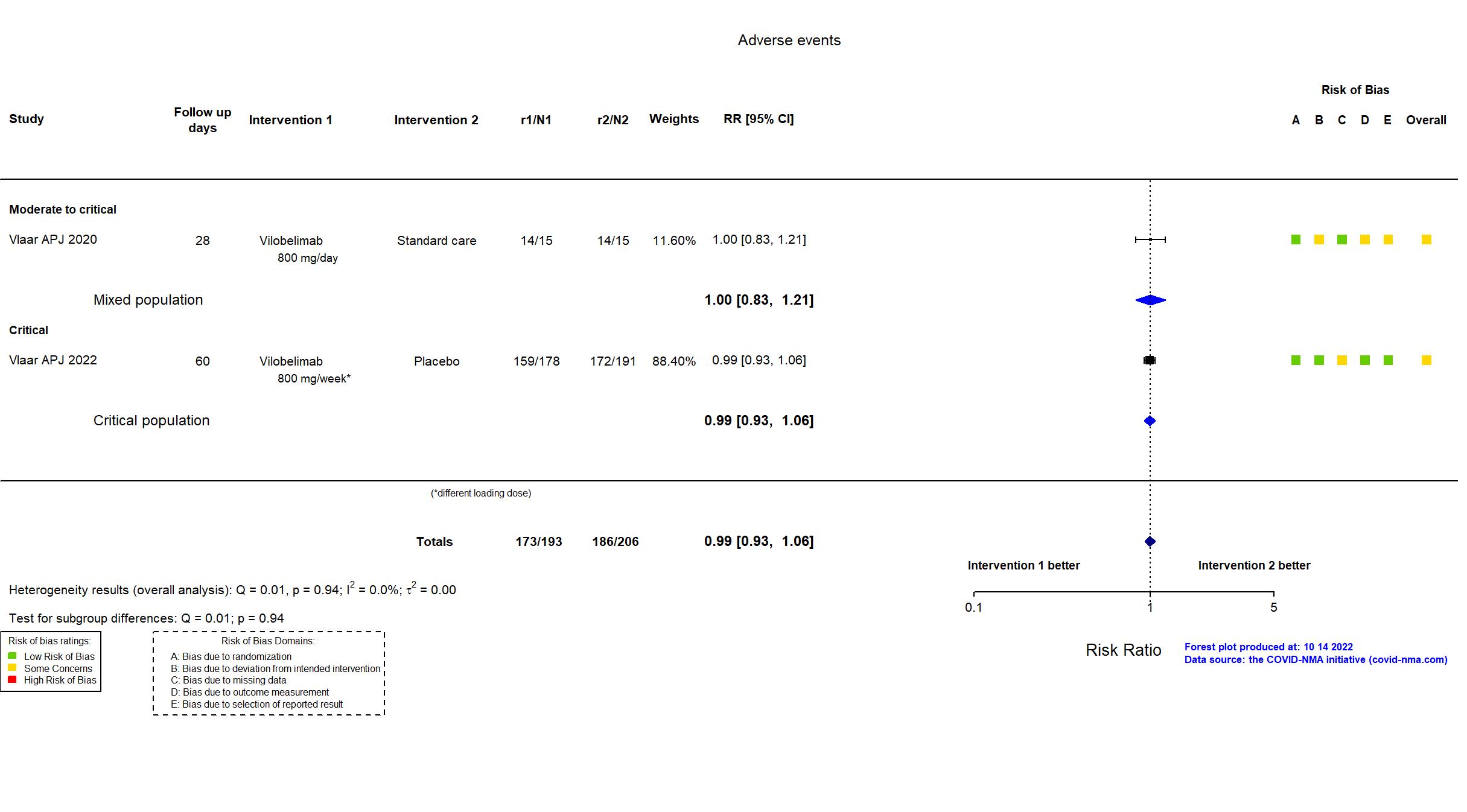
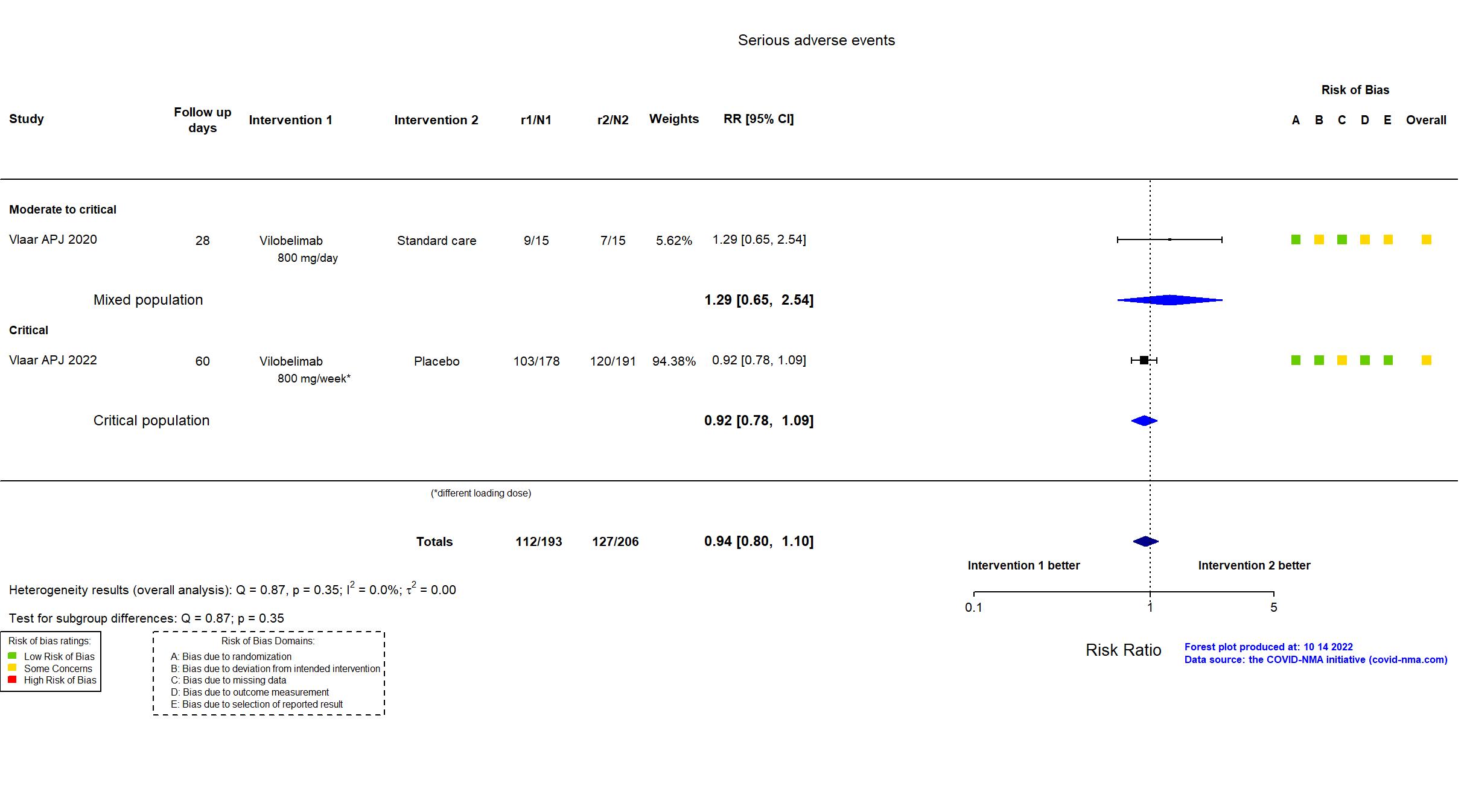
| Randomized participants : Vilobelimab=178 Placebo=191 | |
| Characteristics of participants N= 369 Mean age : NR 252 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=0 Critical: n=369 | |
| Primary outcome | |
| In the register Mortality [Time Frame: Day 28]: 28-day all-cause mortality | |
| In the report All-cause mortality at 28 days in the full analysis set | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: N |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article and its supplement, the protocol, SAP and study registry were used in data extraction and risk of bias assessment. The study (N=369) achieved the target sample size specified in the trial registry. There is no change from the trial registration in the intervention and control treatments. The registry primary outcome reflects the reported primary outcome. Adverse and serious adverse events were classified as "treatment-emergent" in the report. |
Trial NCT04333420
Publication PANAMO - Vlaar APJ, Lancet Rheumatol (2020) (published paper)
Dates: 2020-03-31 to 2020-04-24
Funding: Private (InflaRx GmbH)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / The Netherlands Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Vilobelimab 800 mg IV on days 1, 2, 4, 8 and 15. Two additional doses could be administered (one between days 11 and 13, one at day 22) depending on the patient's condition. |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Standard care=15 Vilobelimab=15 | |
| Characteristics of participants N= 30 Mean age : NR 22 males Severity : Mild: n=0 / Moderate: n=4 / Severe: n=8 Critical: n=18 | |
| Primary outcome | |
| In the register Change in PaO2/FiO2 [ Time Frame: Baseline to Day 5 ] Relative change (%) from baseline in Oxygenation Index (PaO2 / FiO2) to day 5. | |
| In the report percentage change in PaO2/FiO2 in the supine position from baseline (day 1, before study drug administration and within 1 h before or after randomisation) to day 5 | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: N |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to all available versions of the published/preprint article, the study registry was used in data extraction and risk of bias assessment. This report is based on the results of a phase II trial; the sample size specified in the registry was not used "This was planned to inform the choice of endpoints and study population specifications for a potential phase 3 part of the study. For phase 2, we pragmatically set the sample size to 30 patients". There is no change from the trial registration in the intervention and control treatments. There is no change from the trial registration in the primary outcome however, mortality was not an outcome listed in the trial registry. Authors state in the discussion the limitations of the primary outcome reporting in the trial registry and say that even though it did not show a difference, treatment showed a lower rate in mortality, "which is consistent with a potential treatment benefit of IFX-1". Serious adverse events and total averse events were also not listed in registry. The study was funded by the drug company who made the drug, and they were also part of the authorship team. |