Lopinavir + Ritonavir vs Standard care (RCT)
Mild outpatients
FOREST PLOTS -2022-04-15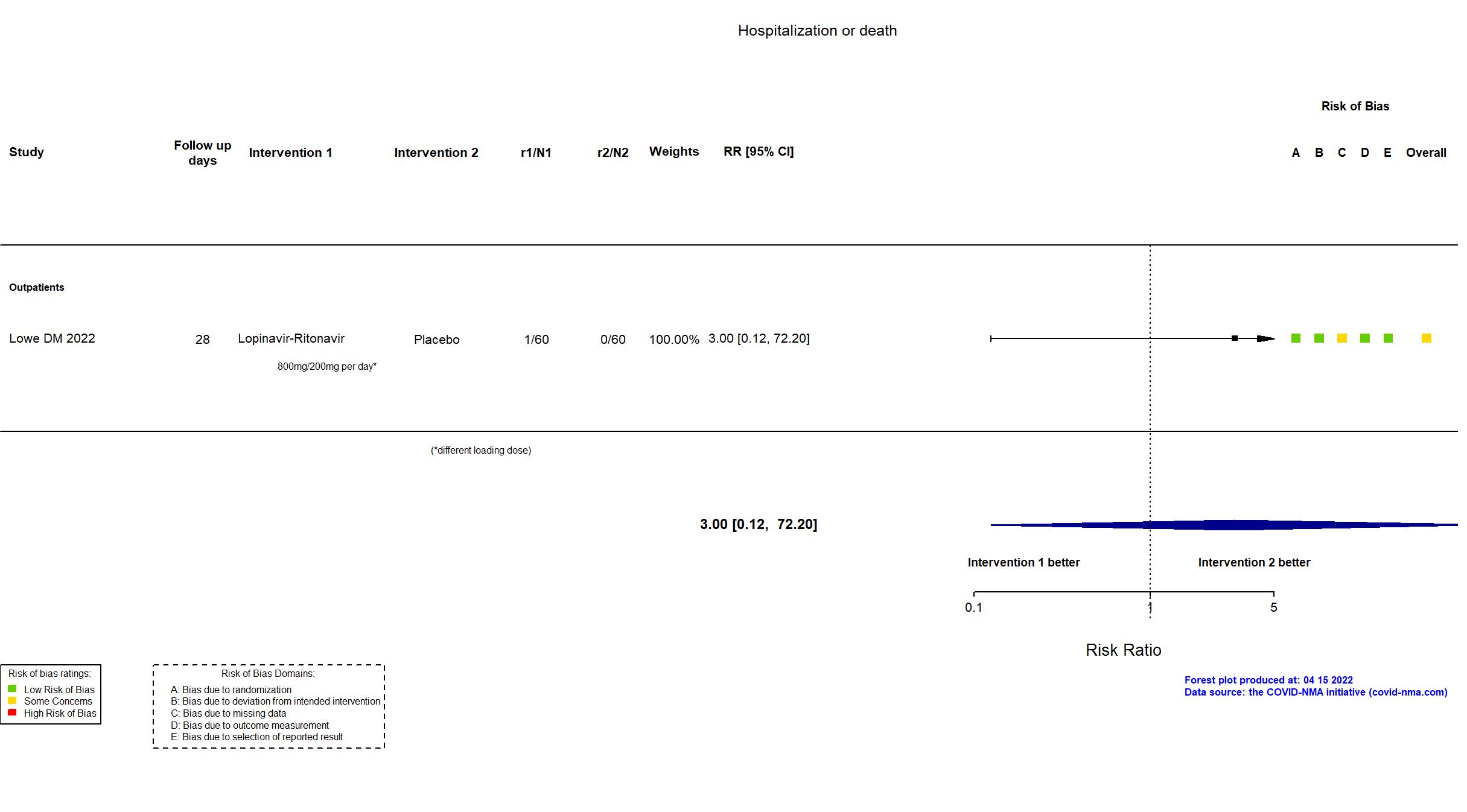







Trial NCT04499677
Publication FLARE - Lowe DM, PLoS Med (2022) (published paper)
Dates: 2020-10-06 to 2021-11-04
Funding: Mixed (LifeArc, UK; Fujifilm Toyama Chemical Co. provided favipiravir and favipiravir placebo free of charge.)
Conflict of interest: Yes
| Methods | |
| RCT Blinding: double blinding | |
| Location :
Multicenter / UK Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
LPV/r+FAV Initial dose: 1800 mg FAV + 400/100 mg LPV/r orally twice a day on Day 1 -Maintenance dose: 400 mg FAV + 200/50 mg LPV/r orally 4 times a day on Days 2-7. Lopinavir-Ritonavir Initial dose: 400/100 mg LPV/r orally twice a day on Day 1 -Maintenance dose: 200/50 mg LPV/r orally 4 times a day on Days 2-7. Favipiravir Initial dose: 1800 mg FAV orally twice a day on Day 1 -Maintenance dose: 400 mg orally 4 times a day on Days 2-7. |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=60 LPV/r+FAV=61 Lopinavir-Ritonavir =60 Favipiravir=59 | |
| Characteristics of participants N= 240 Mean age : NR 123 males Severity : Mild: n= 239/ Asymptomatic: n=1 | |
| Primary outcome | |
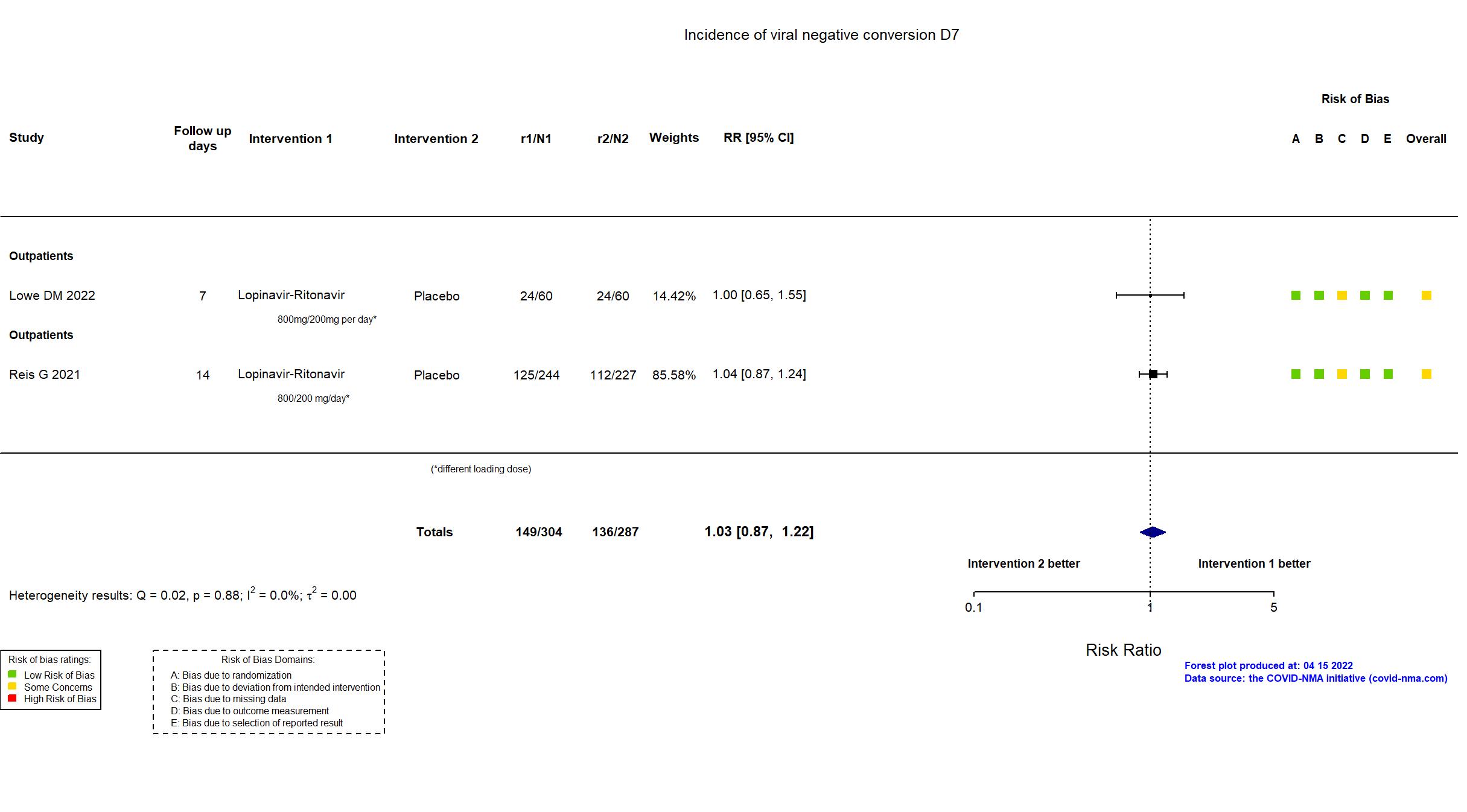
| In the register Upper respiratory tract viral load at Day 5 [ Time Frame: Day 5 from randomisation ] Quantitative polymerase chain reaction (PCR) performed on saliva samples at Day 5 of therapy | |
| In the report Viral load measured by quantitative polymerase chain reaction (PCR) performed on saliva samples at Day 5 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the published article, the pre-print article, protocol, statistical analysis plan and prospective study registry were used in data extraction and risk of bias assessment. There is no change from the trial registration in the intervention and control treatments. The registry primary outcome reflects the reported primary outcome. The study (n=240) achieved the target sample size (n=240) specified in the trial registry.
This study was updated on August 8th, 2022 with data obtained from contact with authors. This study was updated on January 19th, 2023 with data extracted from the published report. |
Trial NCT04403100
Publication TOGETHER - Reis G, JAMA (2021) (published paper)
Dates: 2020-06-02 to 2020-10-09
Funding: Public/non profit (Bill and Melinda Gates Foundation)
Conflict of interest: No
| Methods | |
| RCT Blinding: | |
| Location :
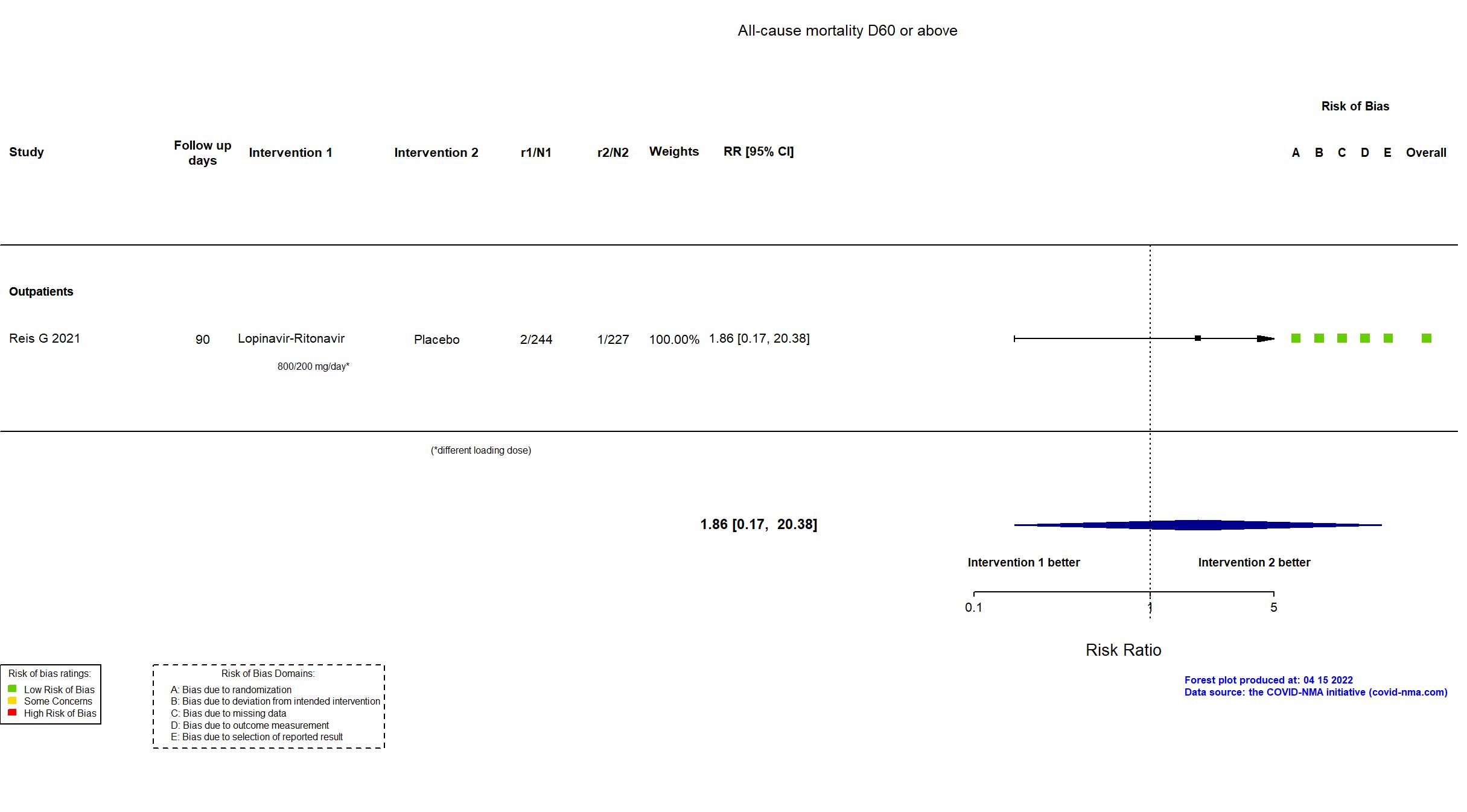
Multicenter / Brazil Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Hydroxychloroquine Initial dose: 800 mg orally once-off - Maintenance dose: 400 mg orally once per day for 9 days Lopinavir-Ritonavir LPV/r: Initial dose 800/200 mg orally twice on day 1 - Maintenance dose: 400/100 mg orally every 12 hours for 9 days |
|
| Control
Placebo | |
| Participants | |
| Randomized participants : Placebo=227 Hydroxychloroquine=214 Lopinavir-Ritonavir =244 | |
| Characteristics of participants N= 685 Mean age : NR 308 males Severity : Mild: n= 685/ Asymptomatic: n=0 | |
| Primary outcome | |
| In the register Proportion of participants who were hospitalized for progression of COVID-19 disease [ Time Frame: Measuring during 28-day period since randomization (Intention to treat analysis) ]; Proportion of participants who died due to COVID-19 progression and/ or complications [ Time Frame: Measuring during 28-day period since randomization (Intention to treat analysis) ] | |
| In the report COVID-associated hospitalization and death | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the study registry and protocol were used in data extraction and risk of bias assessment. Enrollment into the hydroxychloroquine and lopinavir-ritonavir groups was discontinued in this platform study based on a decision from the independent Safety Monitoring Board following interim analysis results showing that it would be clinically irrelevant to recommend either treatment. The longest follow up in the report was 90 days, and in the registry 28 days. A number of outcomes in the registry were not reported (need for mechanical ventilation, shock and need for vasoactive amines, death due to pulmonary or cardiovascular complications). The definition used in the time to symptom resolution outcome in the paper (resolution of combined symptoms using the WURSS scale) was not pre-specified in the registry [Time to clinical improvement - Proportion of participants with clinical improvement, defined as normalization of temperature, Respiratory rate, SaO2, and cough relief (> 50% compared to baseline measured on a visual analog scale) in the last 72 hours] or in the protocol [Time until clinical improvement (up to 28 days), defined normalization of temperature, respiratory rate, SaO2, and cough relief (> 50 in relation to baseline measured on a visual analog scale) in the last 72 hours; Reduction in the perception of dyspnea (upper respiratory tract respiratory symptoms scale - WURSS-11) on days 0, 3, 7, 14 and 28 days)]. |