Lopinavir + Ritonavir vs Standard care (RCT)
Hospitalized patients
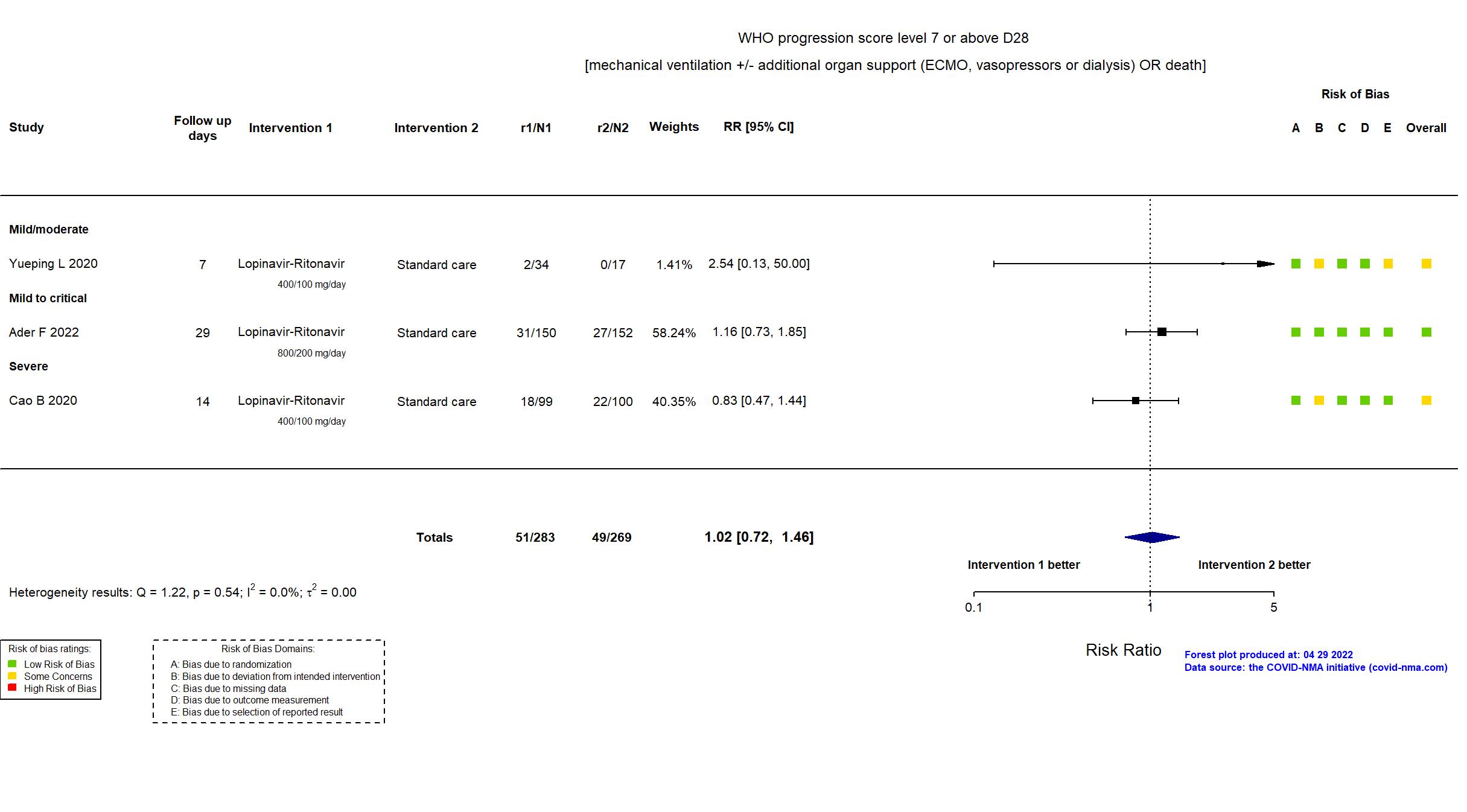
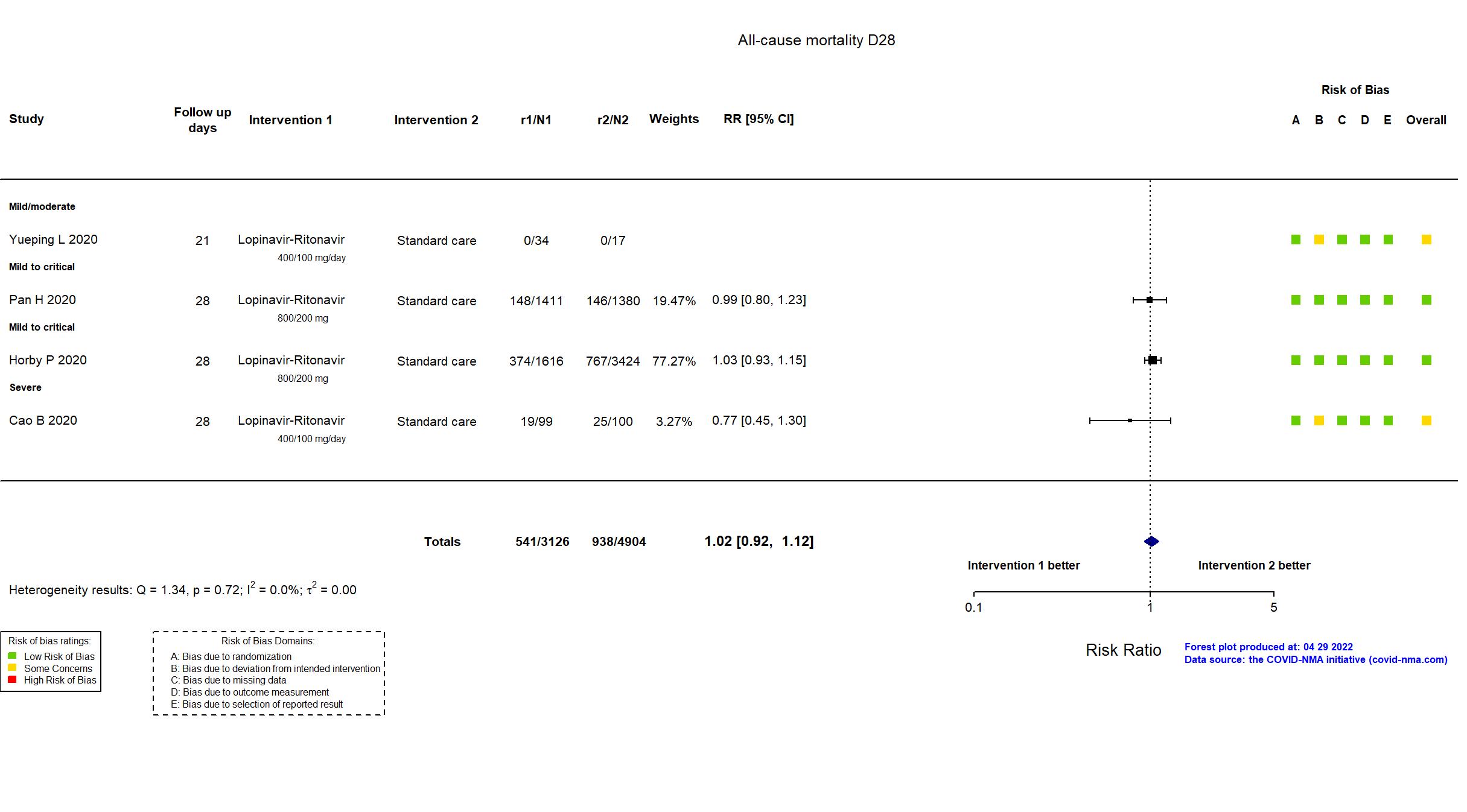
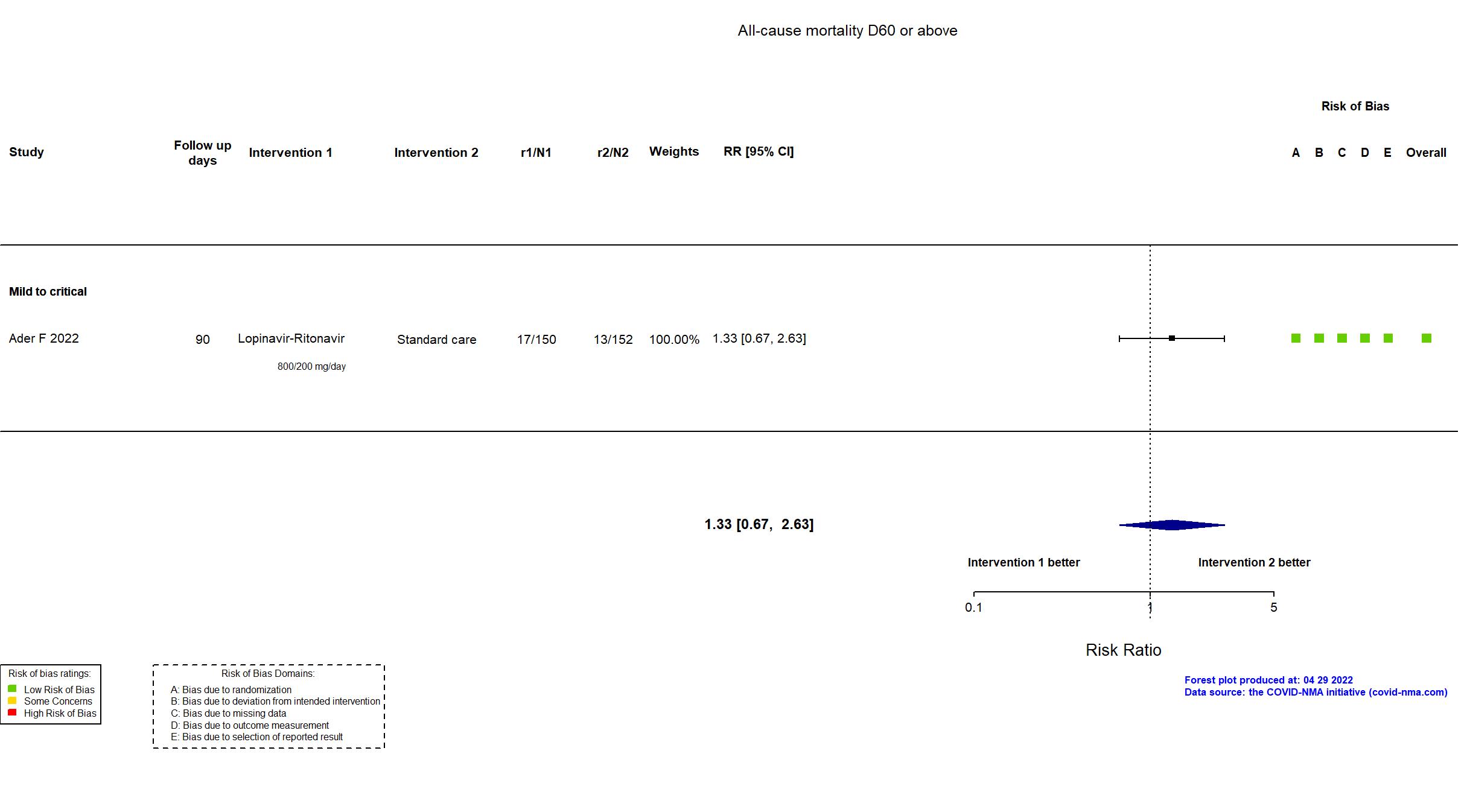
FOREST PLOTS -2022-04-29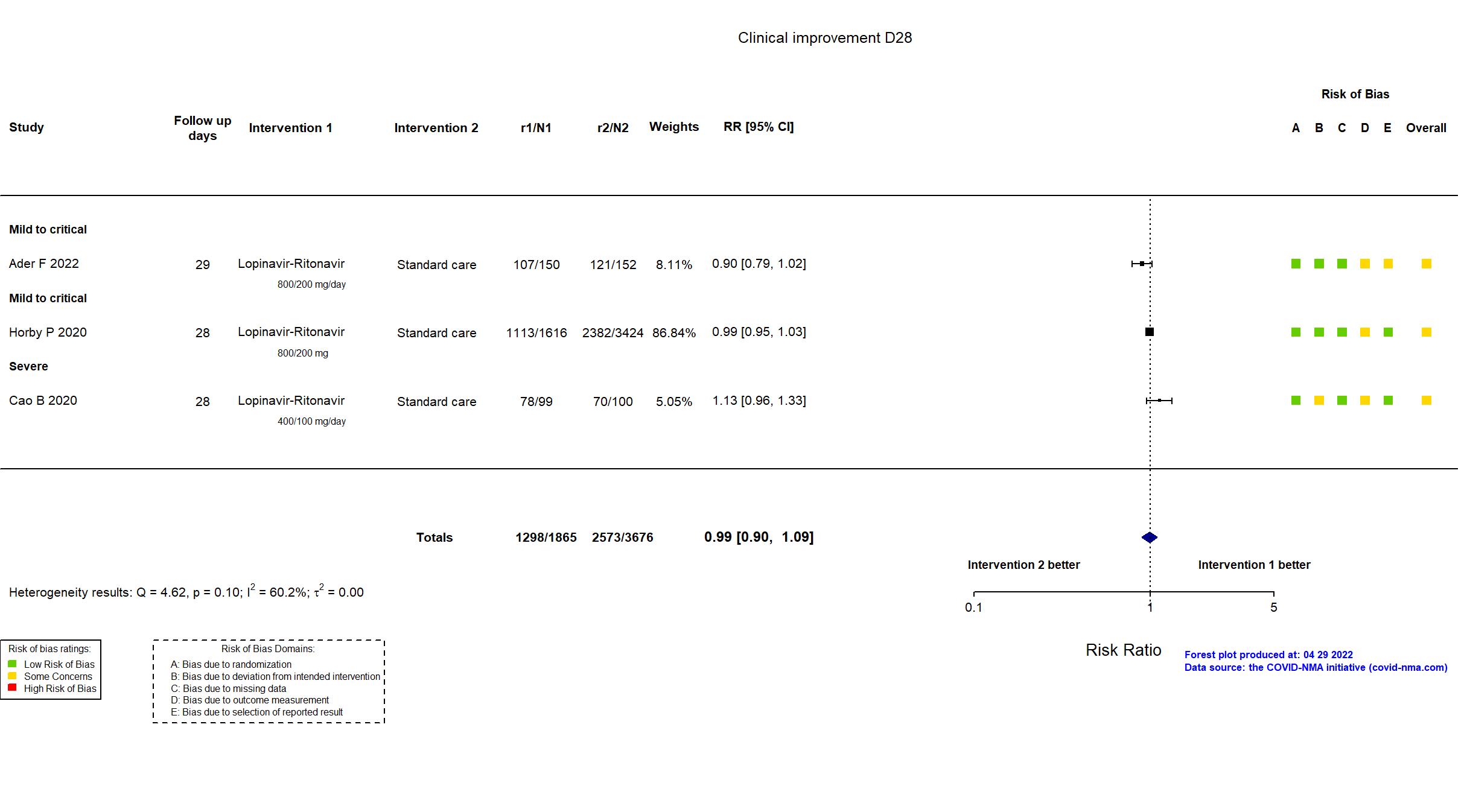

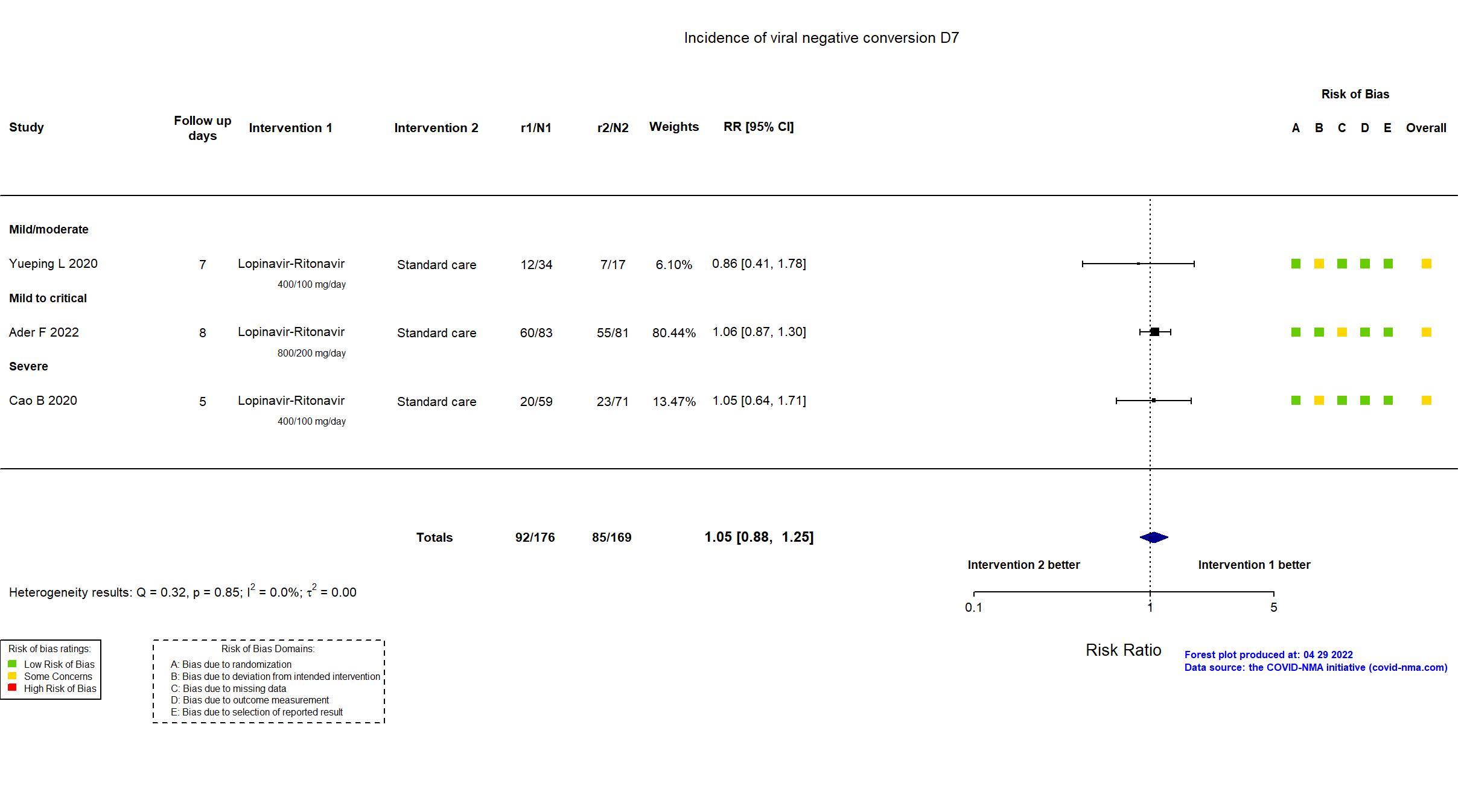
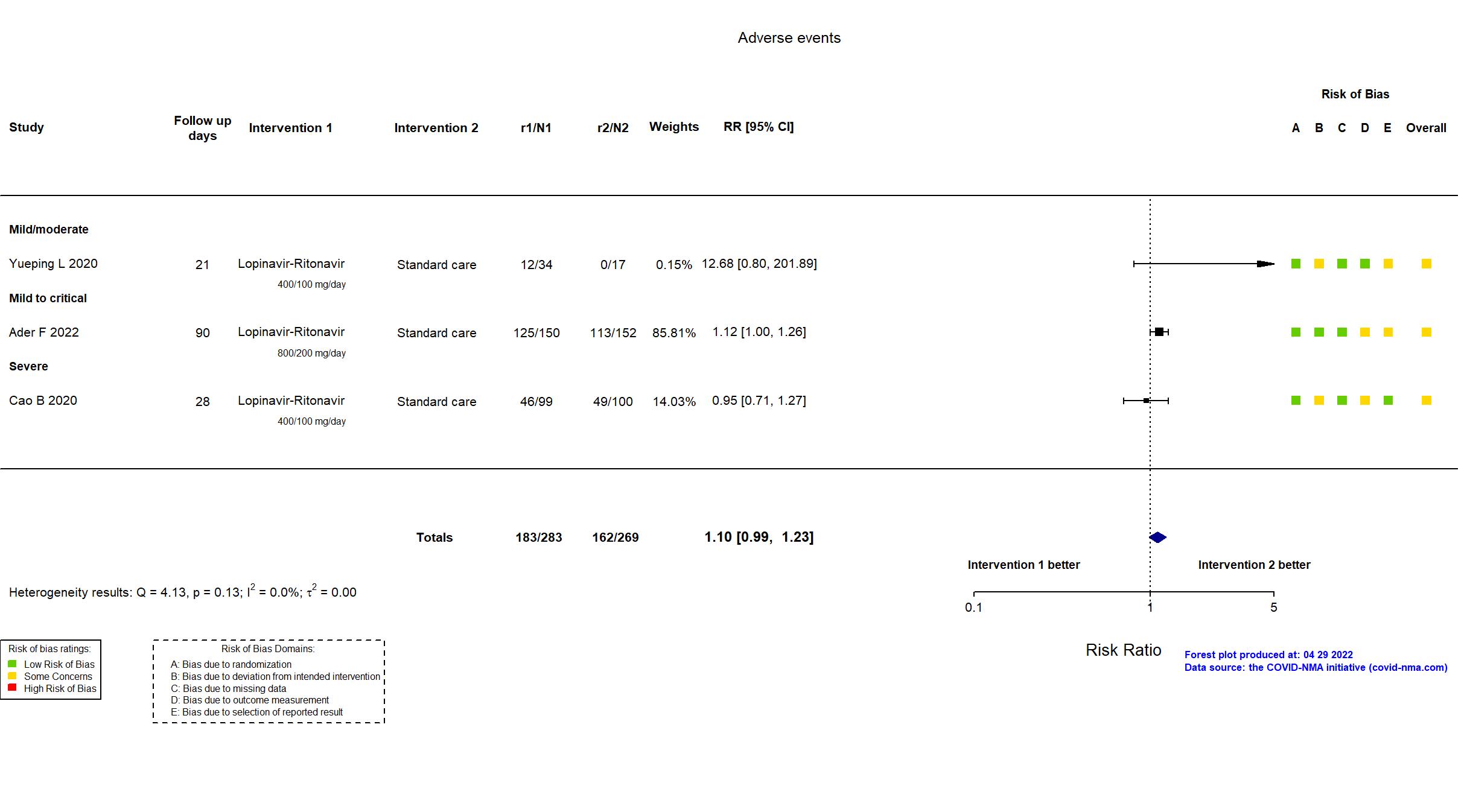
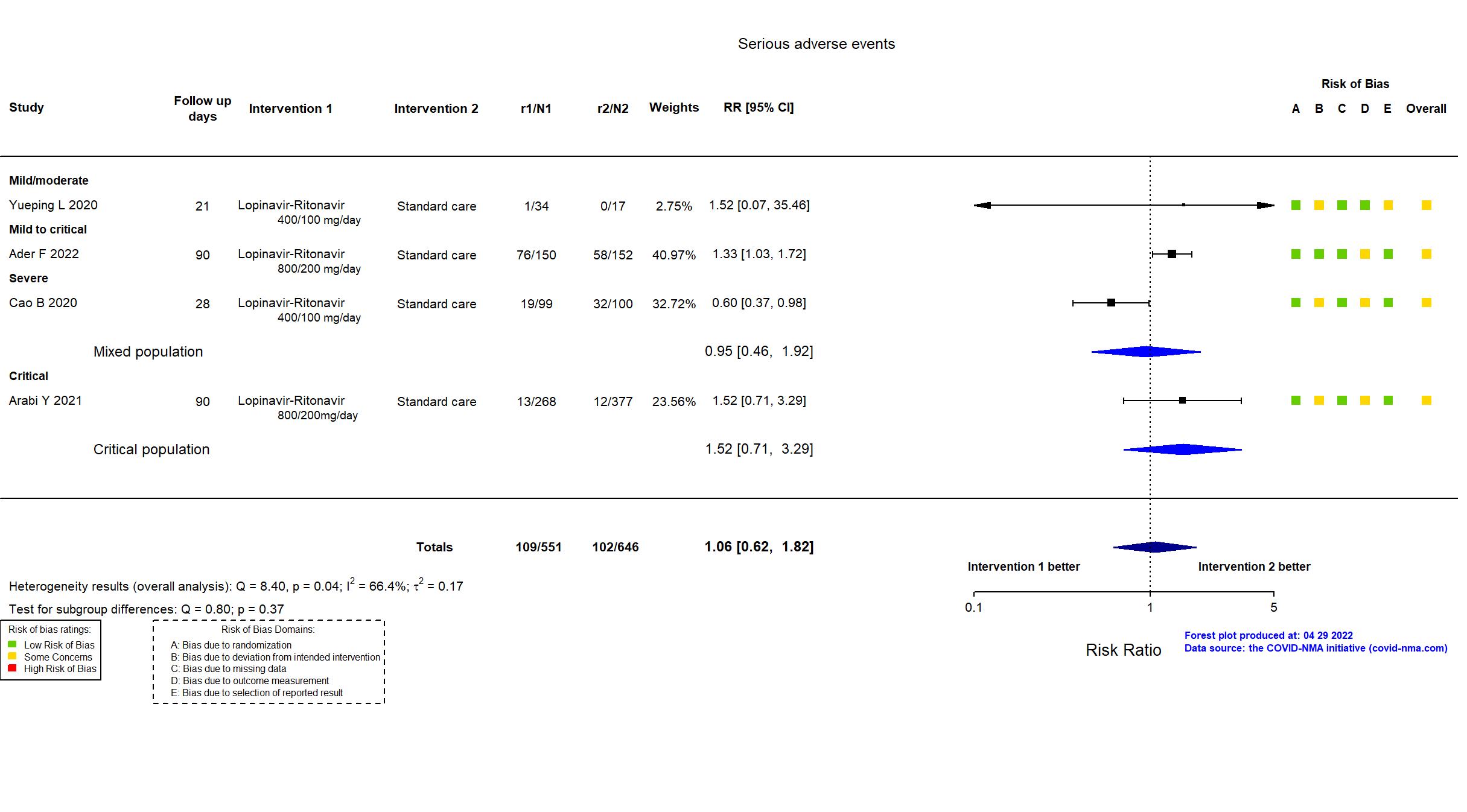
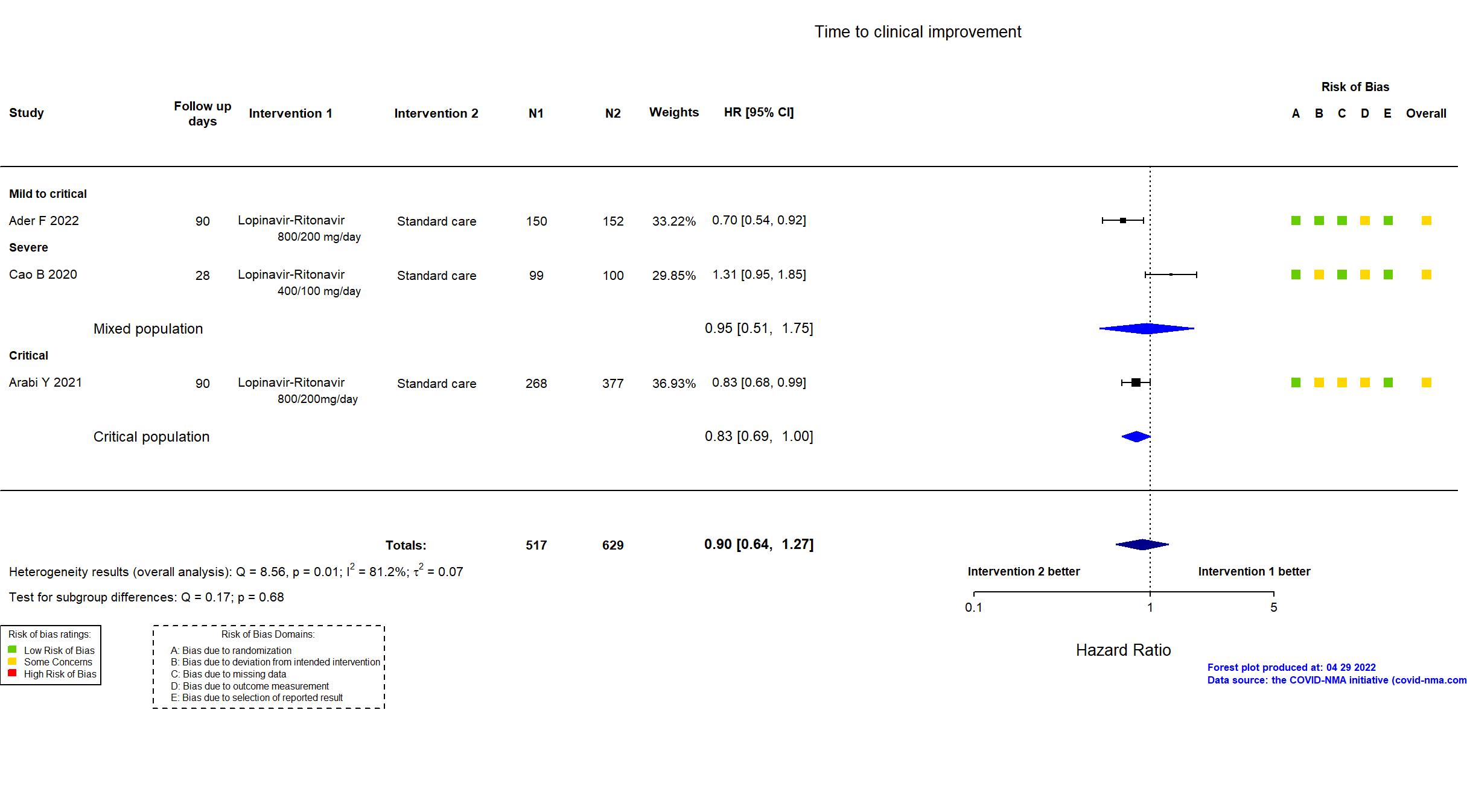










Trial NCT04315948
Publication DisCoVeRy - Ader F, medRxiv (2022) (preprint)
Dates: 2020-03-22 to 2020-06-29
Funding: Mixed (Programme Hospitalier de Recherche Clinique, DIM One Health Île-de-France, REACTing, INSERM. GILEAD, SANOFI, MERCK and ABBVIE (drug provision) )
Conflict of interest: Yes
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / France, Luxembourg Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Lopinavir-Ritonavir Lpv/R: 400/100 mg orally or by nasogastric tube every 12h for 14 days LPV/r+IFN beta-1a Lpv/R: 400/100 mg orally or by nasogastric tube every 12h for 14 days IFN beta-1a: 44 mcg subcutaneously on days 1, 3 and 6 Hydroxychloroquine 400 mg orally twice on day 1 then 400 mg once daily for 9 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Lopinavir-Ritonavir=150 LPV/r+IFN beta-1a=150 Hydroxychloroquine=151 Standard care=152 | |
| Characteristics of participants N= 603 Mean age : NR 421 males Severity : Mild: n=21 / Moderate: n=354 / Severe: n=62 Critical: n=156 | |
| Primary outcome | |
| In the register Percentage of subjects reporting each severity rating on a 7-point ordinal scale [ Time Frame: Day 15]; a. Not hospitalized, no limitations on activities; b. Not hospitalized, limitation on activities; c. Hospitalized, not requiring supplemental oxygen; d. Hospitalized, requiring supplemental oxygen; e. Hospitalized, on non-invasive ventilation or high flow oxygen devices; f. Hospitalized, on invasive mechanical ventilation or ECMO; g. Death | |
| In the report Clinical status at day 15 as measured on the 7-point ordinal scale of the WHO Master Protocol (v3.0, March 3, 2020) | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment |
In addition to the journal/pre-print article and its supplementary materials, the study registry and published protocol were used in data extraction and risk of bias assessment.
DisCoVeRy is an adaptive, add-on trial of the WHO Solidarity trial. The same treatments are evaluated in DisCoVeRy and in Solidarity. Solidarity has three endpoints which are also secondary endpoints of DisCoVeRy: (1) mortality during hospitalization (the primary endpoint of Solidarity), (2) length of hospital stay and (3) time to mechanical ventilation or transfer to intensive care. There were no substantive differences between the protocol, registry and publication/pre-print in study population, procedures, interventions or outcomes. The 3 intervention arms presented here were terminated early. Quote: "The present analysis is based on the protocol v7.0 of April, 5th 2020,(14) with two secondary outcomes added in protocol v9.0 of June, 29th 2020...On May 25th 2020, following a safety warning on hydroxychloroquine use(19), enrollment in the hydroxychloroquine arm was suspended at the request of the French Agency of drug Security (Agence Nationale de Sécurité du Médicament). On June 13th, based on the interim analysis of the Solidarity data, the Solidarity and DisCoVeRy trial DSMBs recommended to definitely stop the hydroxychloroquine arm due to futility. This decision was endorsed by the DisCoVeRy steering committee on June 17th. The Solidarity DSMB advised to stop the lopinavir/ritonavir arm due to futility on June, 23th. Thereafter, the DisCoVeRy DSMB further advised to stop both the lopinavir/ritonavir-containing arms due to additional safety concern on June, 25th. This decision was endorsed by the DisCoVeRy steering committee on June 27th with subsequent interruption on June, 29th." The pre-planned remdesivir arm is continuing and currently enrolling. This was substantially underpowered as <25% of the pre-stated sample size was randomized. On 1st of May, 2021, this study was updated based on the published report. On 26th of April, 2022, this study was updated based on the preprint reporting final results. |
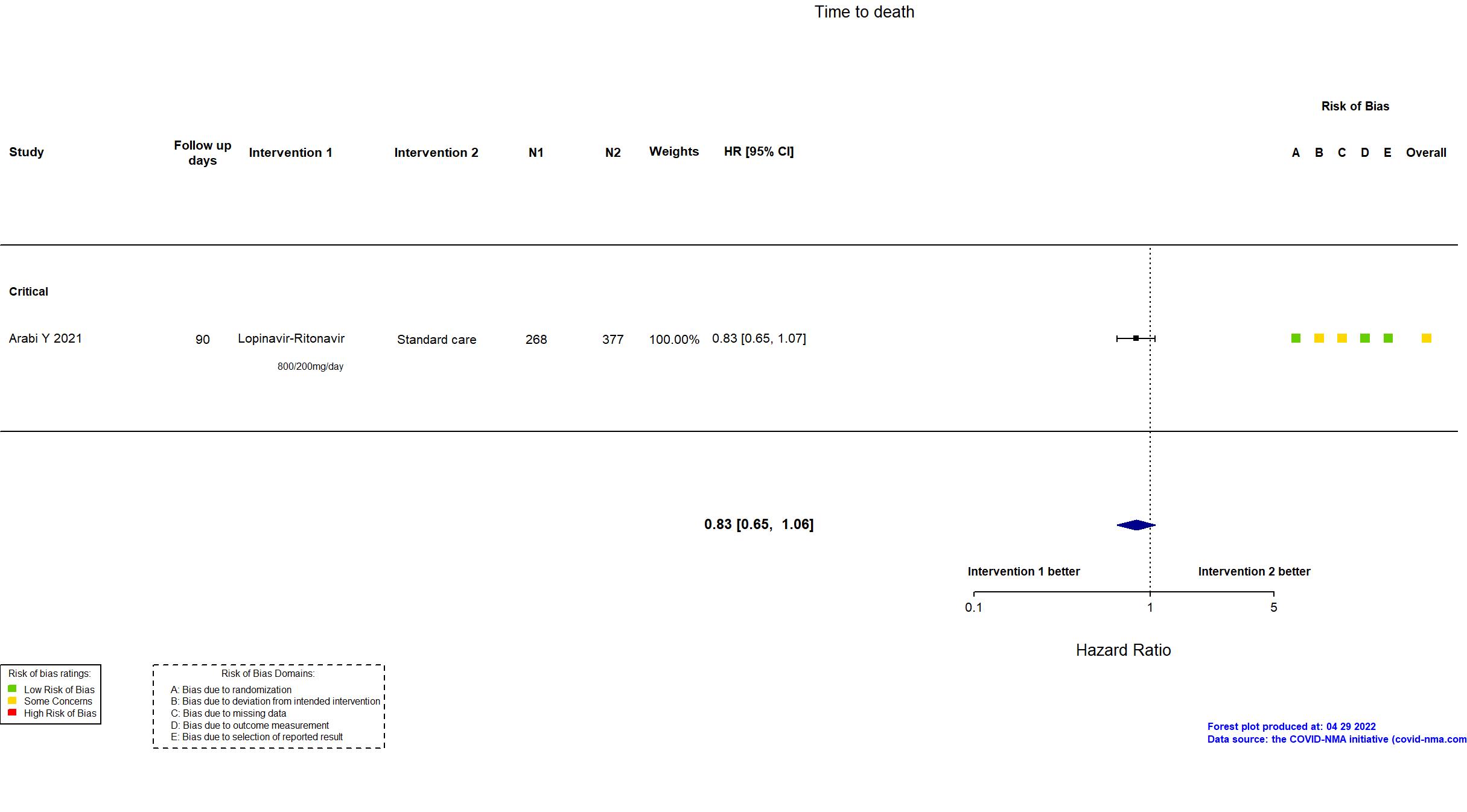
Trial NCT02735707
Publication Arabi Y, Intensive Care Med (2021) (published paper)
Dates: 2020-04-08 to 2020-11-19
Funding: Mixed (The European Union through the Platform for European Preparedness Against emerging Epidemics (PRE‑PARE) consortium and Horizon 2020 research and innovation program (the Rapid European Covid-19 Emergency Research response (RECOVER) consortium; the Australian National Health and Medical Research Council; the Health Research Council of New Zealand; Canadian Institutes of Health Research Strategy for
Patient-Oriented Research Innovative Clinical Trials Program Grant; the U.K. NIHR and the NIHR Imperial Biomedical Research Centre; the Health Research Board of Ireland; the UPMC Learning While Doing Program; the Breast Cancer Research Foundation; the French Ministry of
Health; the Minderoo Foundation; Amgen; Eisai; the Global Coalition for Adaptive Research; the Wellcome Trust Innovations Project)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Australia, Canada, France, Germany, Ireland, Netherlands, New Zealand, Portugal, Saudi Arabia, UK, USA Follow-up duration (days): 90 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Lopinavir-Ritonavir 400 mg + 100 mg orally twice daily for 5-14 days or ICU discharge Hydroxychloroquine Initial dose: two oral 800 mg doses 6 h apart - Maintenance dose: 400 mg orally twice daily for 6 days HCQ+LPV/r 400 mg Lopinavir + 100 mg Ritonavir orally twice daily for 5-14 days or ICU discharge + HCQ two 800 mg oral doses 6 h apart, then 400 mg orally twice daily for 6 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Lopinavir-Ritonavir=268 Hydroxychloroquine=52 HCQ+LPV/r=29 Standard care=377 | |
| Characteristics of participants N= 726 Mean age : NR 488 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=0 Critical: n=694 | |
| Primary outcome | |
| In the register All-cause mortality [ Time Frame: Day 90 ]; Days alive and not receiving organ support in ICU [ Time Frame: Day 21 ] | |
| In the report composite ordinal scale of the number of respiratory and cardiovascular organ support-free days (OSFD) and in-hospital mortality with death assigned the worst outcome, up to day 21 | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to the published article, the supplementary appendix with protocol and statistical analysis plan, and study registry were used in data extraction and risk of bias assessment. This was a multi-country adaptive platform trial (REMAP-CAP) with no pre-defined sample size. This paper reported on critically ill patients and four of the trial's intervention arms. The interventions arms reported were added to the registry (15 April 2020) one week after the start of recruitment to the arms (8 April 2020), but the domain-specific protocol amendment was dated before start of recruitment (1 April 2020). There were no substantive differences between the published article and the registry, protocol and statistical analysis plan in population, procedures, interventions and outcomes. Enrollment into the hydroxychloroquine and combination therapy study arms was halted before reaching any pre-specifed internal trigger but based on external evidence, consequently, these arms had much smaller sample sizes compared to the other arms. |
Trial ChiCTR2000029308
Publication Cao B, N Engl J Med (2020) (published paper)
Dates: 18jan2020 to 03feb2020
Funding: Mixed (Major Projects of National Science and Technology on New Drug Creation and Development; Chinese Academyof Medical Sciences (CAMS) Emergency Project of Covid-19; National Science Grant for DistinguishedYoung Scholars.
Dr. Jaki is a recipient of)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Single center / China Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Lopinavir-Ritonavir LPV/r: 400mg and 100mg orally twice a day for 14 days. |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Lopinavir-Ritonavir=99 Standard care=100 | |
| Characteristics of participants N= 199 Mean age : NR 120 males Severity : Mild: n=0 / Moderate: n=0 / Severe: n=199 Critical: n=0 | |
| Primary outcome | |
| In the register Clinical improvement time of 28 days after randomization; The 7-point scale; 7 points: death; 6 points: admission to ECMO and / or mechanical ventilation; 5 points: Hospitalized for non-invasive ventilation and / or high-flow oxygen therapy 4 points: hospitalization for oxygen therapy 3 points: Hospitalization does not require oxygen therapy 2 points: discharged but not restored to normal functional status 1 point: discharged to normal function | |
| In the report Time to clinical improvement, defined as the time from randomization to an improvement of two points (from the status at randomization) on a seven-category ordinal scale or live discharge from the hospital, whichever came first | |
| Documents avalaible |
Protocol Yes. In language other than English Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to all available versions of the published/pre-print article, the study registry and protocol were used in data extraction and risk of bias assessment. The protocol, which was available only in Chinese, was examined by native speakers. The study achieved the target sample size specified in the trial registry. There is no change from the trial registration in the intervention and control treatments. The primary outcome indicated in registry reflects the primary outcome reported in the paper. Some outcomes from the registry are not reported in the paper (e.g., proportion of patients in each category of the 7-point scale on day 28 after randomization). |
Trial NCT04381936
Publication RECOVERY (LPV/r) - Horby P, Lancet (2020) (published paper)
Dates: 2020-03-19 to 2020-06-29
Funding: Mixed (NIHR Oxford Biomedical Research Centre; Wellcome Trust; Bill and Melinda Gates Foundation; Health Data Research UK; UK Department for International Development; NIHR Health Protection Unit in Emerging and Zoonotic Infect)
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / UK Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Lopinavir-Ritonavir lopinavir 400 mg plus ritonavir 100 mg orally every 12 hours for 10 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Lopinavir-Ritonavir=1616 Standard care=3424 | |
| Characteristics of participants N= 5040 Mean age : NR 3077 males Severity : Mild: n=1321 / Moderate: n=* / Severe: n=* Critical: n=204 | |
| Primary outcome | |
| In the register All-cause mortality [Time Frame: Within 28 days after randomisation] | |
| In the report 28-day all-cause mortality | |
| Documents avalaible |
Protocol Yes. In English Statistical plan Yes Data-sharing willing stated in the publication:
|
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to all available versions of the published article, the study registry, statistical analysis plan, supplementary appendix and protocol were used in data extraction and risk of bias assessment. This trial is part of a large ongoing study with comparisons of multiple treatments for COVID-19 with standard care. As of October 2020 the trial continues to study the effects of azithromycin, tocilizumab, convalescent plasma, and REGN-CoV2. Recruitment to the lopinavir–ritonavir arm was terminated because the independent data monitoring committee, chief investigators and steering committee concluded that the data showed no beneficial effect of lopinavir–ritonavir in patients admitted to hospital with COVID-19. There were no substantive changes in treatments or outcomes between the published article and study registries, the trial protocol and statistical analysis plan. |
Trial ISRCTN83971151; NCT04315948
Publication SOLIDARITY (LPV/r) - Pan H, N Engl J Med (2020) (published paper)
Dates: 2020-03-22 to 2020-10-04
Funding: Mixed (World Health Organization; Abbvie, Cipla and Mylan (drug donation))
Conflict of interest: No
| Methods | |
| RCT Blinding: Unblinded | |
| Location :
Multicenter / Multinational (30) Follow-up duration (days): 28 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Lopinavir-Ritonavir two lopinavir 200 mg plus ritonavir 50 mg tablets orally twice daily for 14 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Lopinavir-Ritonavir=1411 Standard care=1380 | |
| Characteristics of participants N= 2791 Mean age : NR 1653 males Severity : Mild: n=1067 / Moderate: n=* / Severe: n=* Critical: n=* | |
| Primary outcome | |
| In the register All-cause mortality, subdivided by the severity of disease at the time of randomization, measured using patient records throughout the study | |
| In the report In-hospital mortality | |
| Documents avalaible |
Protocol Yes. In English Statistical plan NR Data-sharing willing stated in the publication: Yes |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Low |
| General comment |
In addition to all available versions of the published manuscript, the pre-print article, the study registry and protocol were used in data extraction and risk of bias assessment. This was a report of interim results of an adaptive trial evaluating 4 drugs: Remdesivir, Hydroxychloroquine, Lopinavir, and Interferon-β1a. No target sample size was pre-specified.
Quote: "The protocol stated “The larger the number entered the more accurate the results will be, but numbers entered will depend on how the epidemic develops… it may be possible to enter several thousand hospitalised patients with relatively mild disease and a few thousand with severe disease, but realistic, appropriate sample sizes could not be estimated at the start of the trial.” The Executive Group, blind to any findings, decided the timing of release of interim results." Quote: "The hydroxychloroquine, lopinavir, and interferon regimens were discontinued for futility on, respectively, June 19, July 4, and October 16, 2020." There was no change from the trial registration in the intervention and control treatments, nor in the primary and secondary outcomes. This study was updated on December 9th using data from the published manuscript. |
Trial NCT04252885
Publication Yueping L, CellPress (2020) (published paper)
Dates: 01feb2020 to 28mar2020
Funding: Public/non profit (Project 2018ZX10302103-002, 2017ZX10202102-003-004 and Infectious Disease Specialty of Guangzhou High-level Clinical Key Specialty (2019-2021) )
Conflict of interest: No
| Methods | |
| RCT Blinding: Participants | |
| Location :
Single center / China Follow-up duration (days): 21 | |
| Inclusion criteria |
|
| Exclusion criteria |
|
| Interventions | |
| Treatment
Lopinavir-Ritonavir LPV/r: 200/50 mg orally twice a day for 7-14 days Umifenovir 200 mg orally 3 times a day for 7-14 days |
|
| Control
Standard care | |
| Participants | |
| Randomized participants : Lopinavir-Ritonavir=34 Umifenovir=35 Standard care=17 | |
| Characteristics of participants N= 86 Mean age : NR 40 males Severity : Mild: n=11 / Moderate: n=75 / Severe: n=0 Critical: n=0 | |
| Primary outcome | |
| In the register The rate of virus inhibition [ Time Frame: Day 0, 2, 4, 7, 10, 14 and 21 ] | |
| In the report Time of positive-to-negative conversion of SARS-CoV-2 nucleic acid from initiating treatment to day 21, with the enrollment day as the first day of treatment | |
| Documents avalaible |
Protocol NR Statistical plan NR Data-sharing willing stated in the publication: No |
| Risk of bias Overall The overall risk of bias reported in the table corresponds to the highest risk of bias for the outcomes assessed for the systematic review |
Some concerns |
| General comment | In addition to all available versions of the published/pre-print article, the study registry and the reply provided by authors were used in data extraction and risk of bias assessment.The study did not achieve the target sample size specified in the trial registry. There is no change from the trial registration in the intervention and control treatments. The primary outcome indicated in the registry is measured at multiple time points, some of which are not reported in the paper. Some outcomes are reported in the paper, but were not pre-specified in the trial registry/protocol (e.g., adverse events). |